Editor’s note: This text-based course is an edited transcript of the webinar, Mechanical Power at a Glance, presented by Keith Lamb, RRT, RRT-ACCS, FAARC, FCCM.
It is recommended that you download the course handout to supplement this text format.
Learning Outcomes
After this course, participants will be able to:
- Define mechanical power its properties
- Describe the literature as it pertains to patient outcomes from excessive mechanical power.
- Explain how to minimize mechanical power applied to the lungs
Ventilator-induced lung injury (VILI), described as such in the literature, stems from our actions or patient-related events. This injury primarily occurs when a patient before you, who is intubated or tracheostomized and under mechanical ventilation, encounters factors often beyond our control—like MVCs, car crashes, viral or bacterial pneumonia, and aspiration incidents. Today, our focus is on areas within our control—ventilator-caused lung injuries.
It is important to understand the differences in those things that you, as a bedside clinician, can do to mitigate or minimize the amount of energy that is applied to the lungs that then, in turn, causes a ventilator-induced lung injury. Once that injury has occurred, no matter what the injury is from, there is an acute release of inflammatory mediators. We will talk more in-depth about that momentarily. Those mediators that are released into the system by that injury circulate systemically. What started in the lungs as a ventilator-induced lung injury then circulates systemically via those inflammatory mediators can cause multi-system organ failure, or those inflammatory mediators go to the liver, the gut, the heart, or the kidney.
What comes next are downstream effects involving other organs. Our prime concern revolves around this subsequent inflammatory reaction, an intensified response to the injury, and the crucial takeaway is that this exacerbation directly worsens outcomes and amplifies mortality rates.
- Ventilator-Induced Lung Injury
- Volutrauma
- Barotrauma
- Atelectrauma
- Biotrauma
- Biotrauma describes a secondary inflammatory response or a biologic response to injury.
- Caused by energy transferred from the ventilator to the lungs.
- Or by the patient P-SILI
For anyone who has responsible for patients under intubation and mechanical ventilation, it is vital to keep these various factors that could harm the patient via the ventilator in mind. The most prevalent concepts we discuss are volutrauma, barotrauma, and atelectrauma. We dive deeper into this shortly, but to offer a general preview, volutrauma denotes the lung stress and strain triggered by excessive tidal volume.
Barotrauma mirrors the lung's exposure to stress and strain under pressure. Atelectrauma signifies the stress and strain the lung undergoes due to the repetitive opening and closing of those alveoli, the delicate single-cell layers. This cycle can ultimately lead to injury or even alveolar fracture.
In the literature, the inflammatory process I previously discussed, should be approached as or leads to biotrauma. This biotrauma constitutes a micro-physiological inflammatory response originating from an injury. We will delve deeper into this shortly, but these are the terms commonly encountered in the literature.
We now explore a deeper understanding of how mechanical power, which is the energy produced by the ventilator, is transferred to the patient. This energy, coupled with the creation of heat, stress, strain, and deformation experienced by the fragile alveoli, eventually culminates in organ failure downstream—a phenomenon referred to as biotrauma in the scientific literature.
Today, we use this term to encapsulate the subsequent inflammatory or biological response to an injury. The patient's DNA and defense mechanisms can partially influence this response. While not entirely equivalent, it bears similarity to someone who gets stung by a bee: one person might feel irritation, while another might face an exaggerated anaphylactic reaction due to their heightened sensitivity to the bee's toxin, potentially leading to a fatal outcome.
A comparable scenario, or something quite akin to it, can be observed in ventilated intubated patients. Some individuals might showcase a substantial reaction to the stress, strain, and energy transmitted by the ventilator, while others may not display such responses. This energy and its corresponding reactions stem from the transfer of energy from the ventilator to the lungs.
As bedside clinicians and educators, we are all aware that positive pressure ventilation, to any degree, is not optimal for patients. It might serve as a temporary life-support measure, but the consequences arising from the energy response or energy transfer to the lungs can be grave. Furthermore, while we will not delve deeply into this aspect today, it is worth noting that the same energy principles—components that explain the details of mechanical power and energy transfer to the lungs—can be encapsulated by a couple of formulas. A relatively newer concept is that the patient's own responses can contribute to this process.
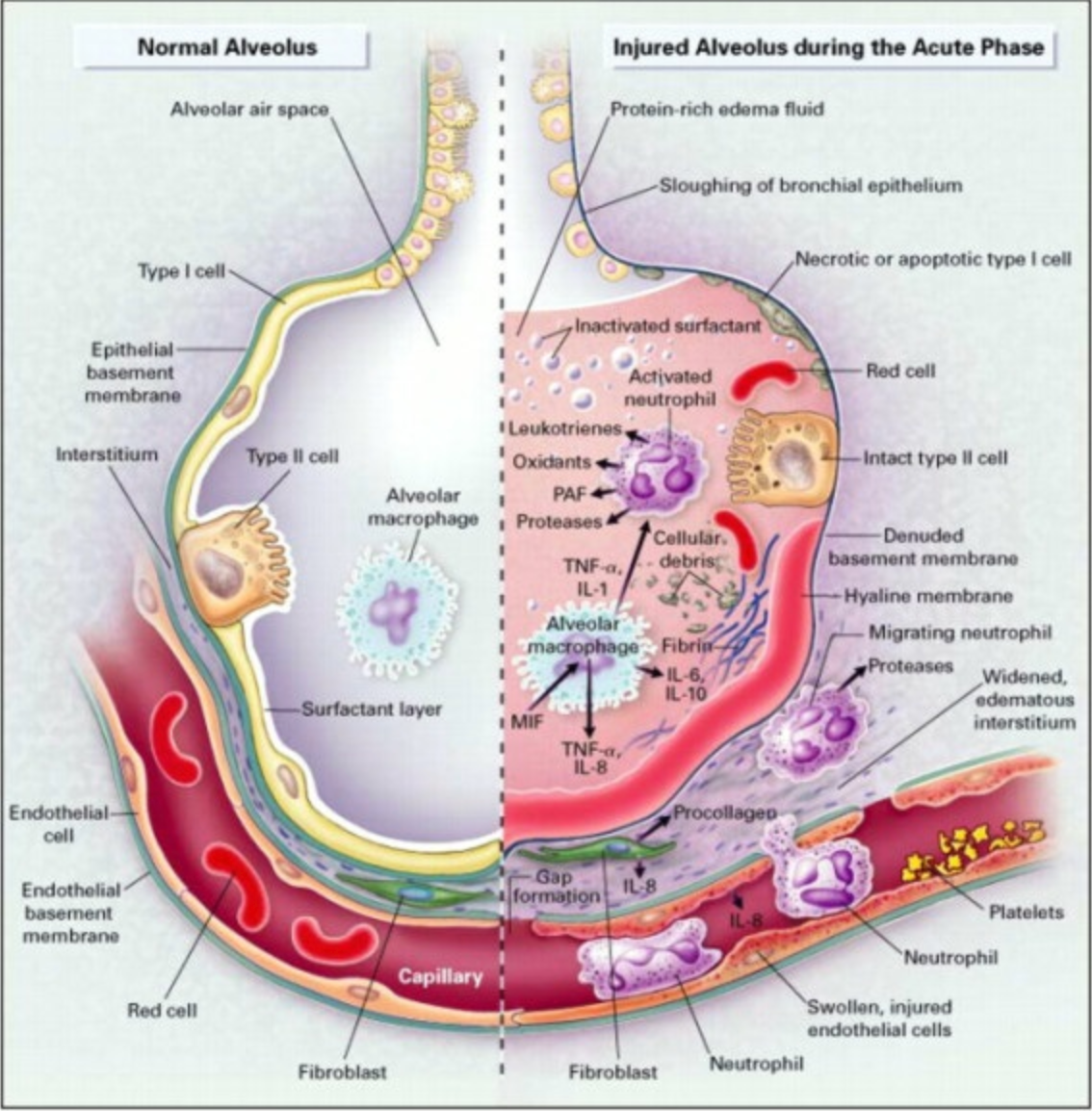
Figure 1. The normal alveolus (Left-Hand Side) and the injured alveolus in the acute phase of acute lung injury and the acute respiratory distress syndrome, Right-Hand Side. (CC BY-SA 4.0 via Wikimedia Commons).
It is fascinating to explore the intricacies of the alveoli and their role in respiratory health. In Figure 1, we have a visual representation that helps us understand the concept of a "sick" alveolus, particularly in the context of Acute Respiratory Distress Syndrome (ARDS). Here is a breakdown of the key features in the illustration:
Healthy Alveolus (Left Side):
- Alveolar Macrophage: This is depicted as a security guard against infections. These cells play a role in the immune response within the alveoli.
- Surfactant Layer: A thin layer of surfactant is shown, responsible for preventing the collapse of the alveolus by reducing surface tension. This is crucial for efficient gas exchange.
- Thin Cellular Structure: The alveolar wall is thin and delicate, enabling efficient gas exchange between the alveolus and the surrounding pulmonary capillaries.
Injured Alveolus (Right Side):
- Injury Response: When the alveolus is injured due to various factors like aspiration, inflammation, or infections, the normal structure undergoes changes.
- Inflammatory Response: Inflammation occurs in response to injury. Alveolar macrophages release pro-inflammatory cytokines, signaling molecules that trigger immune responses.
- Spread of Cytokines: These cytokines cross over from the alveolar capillaries into the bloodstream, affecting various organs and systems throughout the body.
- Neutrophil Infiltration: The cytokines attract neutrophils, a type of white blood cell, into the alveolar interstitium. This response contributes to the inflammation within the lungs.
The concept of biotrauma, a subject we have been delving into extensively, encompasses a secondary inflammatory process triggered by a biological response to injury or a microphysiological reaction to harm. This phenomenon operates at the intricate level of alveoli and within the alveolar-capillary system, intricately avoiding direct visibility. Unlike an evident pneumothorax that can be promptly addressed, biotrauma is not immediately observable. It eludes bedside detection, demanding the deployment of imagination, sophisticated imaging, and clinical insight to effectively manage the intricate transfer of energy from the ventilator to the patient. These inflammatory agents course through the body's intricate systems, implicating other organs, and ultimately culminating in multi-system organ failure. Regrettably, this cascade of events often leads to an amplification of mortality rates. It is worth noting that injuries and inflammation can lead to disruption of the delicate balance within the alveoli, impairing gas exchange and potentially contributing to the development of conditions like ARDS. Understanding these processes is crucial for medical professionals to effectively manage and treat patients with respiratory disorders.
VILI
- Remember that some alveoli are collapsed.
- Some are partially filled with fluid.
- Some are fully functional.
- Alveoli articulate with other alveoli, and what is happening in one alveolus might affect others.
- We refer to this as heterogeneous lung disease.
- One alveolus might “distort” others as they are stretched.
- Some alveoli will be exposed to more or less volume and pressure than others.
To give you a visual, think of the interstitium as the gaps between the alveoli. This is akin to the pleural space. This interstitium serves as a potential area. Typically, nothing interferes with a gas exchange or the permeability of the alveolar-capillary system, allowing for efficient gas exchange. However, when an inflammatory response triggers a release of cytokines – essentially a storm of signaling molecules – the alveolar-capillary membrane system begins to sustain damage. Ultimately, as the integrity of this membrane is compromised, the interstitium and alveoli become flooded with proteinaceous fluid. The once-effective surfactant can no longer maintain alveolar stability.
Consequently, the alveoli collapse due to the absence of surface tension. This dramatic collapse significantly impairs the patient's capacity to exchange gases – a pivotal aspect to consider. Our primary focus lies in understanding the transfer of energy from the mechanical ventilator through the patient's respiratory system.
Then there are those alveoli that remain fully functional. These alveoli untouched by the disease process are impacted directly when volume is introduced. They face the full brunt of stress and strain – terms we will define shortly – from the ventilator. Adding to the complexity, alveoli aren't isolated entities. They are rearranged in clusters, resembling a honeycomb pattern. A fully functional alveolus might neighbor a consolidated one or one that's only partially functioning. it is crucial to grasp this, as it is a reason static measurements on the ventilator might not always be completely reliable. These measurements provide a global pressure, neglecting the interactions within this honeycomb-like network of alveoli. Pressure, volume, stress, and strain experienced by one alveolus can influence another nearby.
The term "heterogeneous lung disease" reflects this complexity, meaning lung pathology is not uniform. Instead, certain areas may differ – dorsal to ventral, apex to base. it is not a consistent picture. Given this, considering that most ARDS cases are heterogeneous, a well-functioning alveolus might inadvertently distort others when overstretched due to pressure, volume, or flow. This might cause it to move away from consolidated alveoli, affecting nearby functional alveoli. This interplay is intricate in microphysiology, but it is essential to bear in mind when caring for these patients.
To put it simply, some alveoli will encounter different amounts of volume and pressure compared to others. Consider this image, which you've likely encountered in your practice. While a standard X-ray might not reveal the full extent of damage, a multidimensional CT scan of the same patient showcases the severity of lung pathology. In comparing the X-ray and the detailed CT slices, you will note that the ventral part of the lungs has some aeration, whereas the dorsal areas seem entirely consolidated. A closer look, however, reveals that certain lung units – individual alveoli – exhibit a mix of both patterns. This is what I mean when I say that numerous alveoli become partially obstructed or consolidated to varying degrees. As they move towards the dorsal regions of the lungs, these alveoli shift into a state of primary consolidation or dysfunction.
The LUNG SAFE Study: A Presentation of the Prevalence of ARDS According to the Berlin Definition!
A few years ago, our team significantly contributed patients to the LUNG SAFE study, conducted by Bellani et al. (2016). This international observational study focused on individuals with ARDS. The study divided participants into two groups: one from the Northern Hemisphere for a few months, and the other from the Southern Hemisphere for a similar duration. Participating healthcare professionals reported their patient data and P/F ratios during this period. The study employed the explicit Berlin definition to assess whether patients met the criteria for ARDS.
We examined a total of 29,144 patients. Upon comprehensive analysis of the collected data, approximately 2,400 of these patients developed ARDS within the initial 48 hours while undergoing invasive mechanical ventilation. When classifying these patients into categories of mild, moderate, and severe ARDS, it is worth mentioning that if you share my perspective – not being the most proficient respiratory therapist, but striving to improve – and if you possess a penchant for literature, you might be aware of the recent redefinition of ARDS.
In contrast to the previous definition, which mandated intubation, a level of PEEP, specific imaging criteria, and a P/F ratio assessment, the new definition takes a different approach. Although that is not our main focus here, I will briefly mention it. The revised definition does not necessarily require intubation, and patients can use a high-flow nasal cannula or non-invasive ventilation. The imaging criteria are more flexible, not exclusively tied to a chest x-ray but extend to other imaging modalities such as ultrasound.
Refocusing on our primary topic, within the cohort of ARDS patients we studied, it was found that 30% had mild ARDS. As a reminder from the Berlin definition, this corresponds to a P/F ratio falling between 200 and 300. Moderate ARDS, delineated by a P/F ratio ranging from 100 to 200, was observed in another group, while severe ARDS, indicated by a P/F ratio below 100, constituted the remaining portion.
The P/F ratio of pO2 to FiO2, offers a straightforward mathematical representation that helps us understand the underlying relationships. To illustrate, consider a scenario where pO2 is 60, and FiO2 is 100%—dividing the pO2 of 60 by the FiO2 of 1.0 results in a P/F ratio of 60.
Delving into the study results, it is noteworthy that 47% of the globally examined ARDS patients fell under moderate ARDS. Among the entire pool of ICU admissions included in the database, 10% of them presented with ARDS. Furthermore, of those patients necessitating mechanical ventilation, 23% were diagnosed with ARDS or fit into its specified classifications.
This presentation aims to convey a vital message from this extensive observational study encompassing 29,000 patients. It emphasizes how sometimes we, as clinicians, may fall short in recognizing evident conditions right before us. Recognition is crucial as it forms the basis for applying the principles of physics and microphysiology in tailoring our mechanical ventilation strategies.
The data underscores this recognition's importance, highlighting that ARDS's clinical awareness stood at 50% for mild cases and 78% for severe instances. This implies that in 20% of cases, severe ARDS went unnoticed. Shockingly, less than two-thirds of all ARDS patients were managed with tidal volumes of eight milliliters per kilogram of predictive body weight or less. Reflecting on the history, we recall the ARDSNet trial 23 years ago, which revealed that employing lower tidal volumes and targeting six milliliters per kilogram of predictive body weight substantially lowered mortality. Yet, even 15 years after this finding, adherence to these practices was lacking.
Exploring another facet of the ARDSNet trial, only 40% of ARDS patients had their plateau pressure measured. Furthermore, a significant 83% of ARDS patients received PEEP levels below 12. Recognizing the impact of PEEP on mechanical power and overall outcomes raises further concerns.
Considering prone positioning, a crucial intervention demonstrated to halve mortality rates in the PROSEVA trial, was astonishingly employed in a mere 16% of severe ARDS cases. Clinician recognition of ARDS correlated with heightened PEEP usage and a greater inclination towards neuromuscular blockade administration. This implies that while our ability to recognize ARDS might be suboptimal, we are more likely to apply appropriate measures once recognized.
Hospital mortality rates for ARDS patients ranged from 35% for mild cases, 40% for moderate cases, and 46% for severe instances. These findings underscore a global phenomenon: irrespective of geographical location, whether it is Charlottesville, Virginia, or diverse regions across the world, the mortality rate for moderate to severe ARDS hovers around 40%. To conclude, ventilator-induced lung injury encapsulates the core theme we have explored.
Biotrauma
The concept of biotrauma, which we have been exploring extensively, encompasses a secondary inflammatory process. It represents a biological response to injury or a microphysiological reaction to such harm. This intricate phenomenon operates within the confines of the alveolar level and the alveolar-capillary system, discreetly eluding direct visibility. It stands in contrast to the more conspicuous pneumothorax, an issue that can be promptly addressed. Biotrauma artfully sidesteps bedside detection, demanding the deployment of imagination, medical imaging, and clinical insight for the adept management of energy transfer from the ventilator to the patient. Within this complex cascade, these inflammatory agents traverse the body's intricate systems, implicating other vital organs. Ultimately, this intricate interplay culminates in multi-system organ failure, a dire outcome that frequently escalates mortality rates.
ARDS Outcomes ARDS (mortality)
Arguably, the most pivotal facet we will delve into pertains to the repercussions stemming from mechanical power or ventilator-induced lung injury. As previously highlighted, regardless of your global location, if a patient grapples with moderate to severe ARDS, their mortality rate typically spans the 40-45% range. Among those afflicted by moderate to severe ARDS, 40-45% do not survive. This juncture underscores its significance: fatalities solely attributed to hypoxemic respiratory failure emerging from severe ARDS are strikingly uncommon.
In fact, only 1 out of 10 ARDS non-survivors succumbs to hypoxemic respiratory failure. This occurrence is remarkably rare. Individuals can tolerate remarkably low pO2 levels. Often, we encounter individuals with severe COPD leisurely traversing malls, their pO2 hovering around 50, yet they persist in their daily routines. it is essential to note that hypoxemia in itself does not bear the sole responsibility for fatality. This uncommon event is so rare that only 1 out of 10 of these patients ultimately succumbs to hypoxemic respiratory failure. A staggering 90%—or 9 out of 10—meet their end due to multi-system organ failure. Grasping the significance of this phenomenon is pivotal, as it unveils a substantial avenue for intervention at our disposal.
Baby Lung
We hold the potential to wield a significant influence in diminishing ventilator-induced lung injury (VILI) by curbing the transmission of mechanical power from the ventilator to the patient. This reduction mitigates the downstream multi-system organ failure, leading to elevated mortality rates. Consider these contrasting chest CT scans of two 5'9" males grappling with considerable respiratory failure. Despite both experiencing severe respiratory failure, the patient on the left showcases less consolidation and alveolar involvement than the patient on the right. Notably, the latter exhibits a pronounced level of alveolar collapse and consolidation.
Now, let's delve into a pivotal question: In a scenario where both patients are 5'9" males, each adhering to a tidal volume of 6 ml/kg of predictive body weight, which patient's lungs would endure more stress? This question spotlights a critical element deserving of attention. When we refer to the predicted tidal volume chart for a 5'9" male and abide by the recommended guideline of employing six milliliters per kilogram of predictive body weight as an initial benchmark, we ascertain a tidal volume of 424 milliliters for each of these patients.
However, it is imperative to grasp that even individuals of the same height can possess varying lung capacities. Take patient A, for instance, who might present diminished alveolar surface area and reduced compliance. Consequently, in this scenario, the operational alveoli in patient A are subjected to elevated stress-strain energy compared to those in patient B, which boasts a more ample capacity.
While I strongly advocate for understanding the significance of the ARDSNet trial and the central role played by tidal volume in patient management, I emphasize that it is not solely the absolute tidal volume that holds influence. Rather, the pivotal factor lies in the interplay between tidal volume and the lung space available.
I hope this rationale resonates clearly. This remains a core principle weaving through today's discussions. This notion forms the essence: individuals do not universally share an identical alveolar surface area to accommodate pressure and volume. Even if both patients receive identical tidal volumes, remember that the chart or electronic medical record—much like the flow sheets employed in our setting—may suggest precise milliliters per kilogram of predictive body weight. While this initial starting point offers value, it is essential to acknowledge that it is not the sole avenue for evaluation.
Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and Acute Respiratory Distress Syndrome (Brower et al. 2000)
Precisely two decades and three years ago, a landmark trial emerged: the Acute Respiratory Distress Syndrome Network's study (Brower et al. 2000) made its debut in the New England Journal of Medicine. In this pivotal randomized controlled trial, patients were divided into two cohorts—one receiving a tidal volume of six milliliters per kilogram of predicted body weight, while the other received twelve milliliters per kilogram. The investigation also diligently tracked static plateau pressures during the course of the study.
Upon scrutinizing the paper, it becomes evident that the patients were randomly assigned to the twelve or six-milliliter groups. There exists an abundance of data here that we won't delve into today. Nonetheless, the findings revealed that the group subjected to lower tidal volumes (6 mLs) exhibited lower mortality rates compared to the 12 mL group, which experienced a mortality rate similar to the average ARDS patient. Even more noteworthy than this observation is the understanding that the individuals in the lower tidal volume group experienced extended periods before succumbing to non-pulmonary organ failures.
A remarkable three days longer, to be precise. This difference bears statistical significance, evident from the extremely low P-value. However, what holds greater importance is that this finding aligns harmoniously with the overarching theme established since the ARDSNet trial: inducing harm to the lungs predisposes patients to the subsequent development of multi-system organ failures. The occurrence of multi-system organ failure exhibits a substantial correlation with mortality. To illustrate this point, consider a patient afflicted by a single-lung failure—an asthmatic serves as a fitting example.
Upon admission, the asthmatic patient struggles with ventilation—intubation ushers in a complex phase of care. The asthmatic often arrives in a hypercarbia state, with CO2 levels potentially nearing 200 and pH levels potentially dropping below seven, say, to 6.9 or even 6.8. Provided we manage to preserve their lungs from ventilator-induced injury, their survival and subsequent discharge from the hospital is feasible. it is not solely the pH levels that pose the predicament; the primary concern is ensuring adequate ventilation. Once ventilation is optimized, pH levels improve, leading to extubation and liberation from interventions like ECMO. This scenario exemplifies a case of single-organ failure. However, the introduction of other organ complications, such as renal or liver failure, inevitably escalates mortality. This phenomenon, we posit, can be attributed, at least in part, to our ventilator management practices.
Another noteworthy aspect of this study underscores that the group subjected to larger tidal volumes—associated with inferior outcomes—exhibited higher mean PaO2 values. This phenomenon highlights that measures implemented to enhance oxygenation do not invariably translate to improved outcomes. A prime illustration of this concept is evident here. The individuals receiving tidal volumes of 12 milliliters per kilogram of predicted body weight demonstrated enhanced oxygenation and inflation. Yet, these markers of improvement were overshadowed by the smaller tidal volume group's superior outcomes and lower mortality.
We hypothesize that this discrepancy can be attributed to the heightened stress-strain energy imposed on the lungs when subjected to larger tidal volumes. Despite potentially improved gas exchange, the deleterious effects of this additional energy ultimately manifest in poorer outcomes.
Driving pressure and survival in the acute respiratory distress syndrome (Amato et al. 2015)
Fast forward to 2015, we encountered the paper titled "Driving Pressure Survival in the Acute Respiratory Distress Syndrome" (Amato et al. 2015). While not a prospective randomized controlled trial, we loosely regard this paper as a landmark due to its initiation of the notion that volume in relation to available space, as opposed to volume alone, might be pivotal. This paper retrospectively examines nine prominent ARDS trials encompassing 3,500 patients. Driving pressure is the focus, defined as the disparity between plateau pressure or static plateau measurement and PEEP.
If your PEEP stands at 10 and your plateau pressure at 20, then your dynamic or static driving pressure is calculated as 10. Upon analyzing these patients, the core of their realization is a discernible correlation: a driving pressure of 15 centimeters of water aligns with increased mortality. When revisiting the diverse ARDS trials—both retrospective and prospective—it becomes evident that a common denominator among the deceased patients is a driving pressure exceeding 15.
- Increasing driving pressure with fixed PEEP leads to higher mortality
- Increasing PEEP with fixed driving pressure has no effect on mortality
- Increasing PEEP leads to a decrease in driving pressure, survival improves
- Significant increase in mortality once driving pressure > 14 cm H2O
Several key insights stem from this trial in terms of interpretation. These insights are gleaned from the cumulative dataset analysis. When dealing with a ventilated and intubated patient, leaving PEEP unchanged but heightening the driving pressure yields unfavorable outcomes. Conversely, elevating PEEP while maintaining a constant driving pressure—unchanged from its initial value—elicits no discernible impact on mortality rates. This equivalence between mortality rates in both scenarios is mirrored within the framework of mechanical power. This brings us to the impending formula explanation.
Additionally, raising PEEP, if it results in a reduction in driving pressure, signifies recruitment of lung capacity. In cases where volume control ventilation is in play, an increase in PEEP coupled with a drop in the difference between plateau pressure and PEEP coincides with enhanced survival rates.
The need to monitor driving pressure emerges as a proxy for assessing mechanical power—essentially, the energy exerted on the lungs. Elevated driving pressure warrants attention. If it surpasses 14 centimeters of water, mortality rates rise, prompting intervention.
Mechanical power, though not delved into with exhaustive formulas, holds significance. The imperative is not universal comprehension of equations but rather an understanding that specific equation components correlate with unfavorable results.
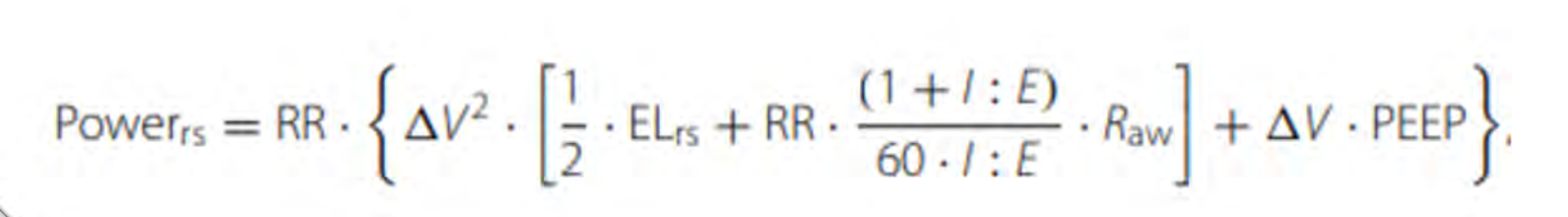
Figure 2. Mechanical Power Equation.
Increasing the power hinges on a quick examination of the equation depicted in Figure 2. The power exerted on the respiratory system can be defined by several factors: the respiratory rate, the alteration in volume (termed delta in volume, corresponding to the tidal volume), the elasticity of the respiratory system, the ratio of inspiration to expiration (I:E ratio), and airway resistance. Notably, airway resistance stands out as a crucial factor—its significance surpasses other elements. Moreover, the change in volume multiplied by PEEP is included, which holds relevance. Any action that heightens or exacerbates these variables invariably results in an increase in power—a concept closely intertwined with ventilator-induced lung injury (VILI). To clarify, the term "power" should immediately evoke thoughts of VILI, and conversely, the term "VILI" should trigger associations with power.
Mechanical power serves as a descriptor for the quantity of energy administered to the lung parenchyma. Another term that might come up is "mechanical energy," often interchangeable with "mechanical work." These equations might be presented in varying formats across different sources—this variability is observable in the equations' structure. However, the takeaway is that within the references provided at the conclusion of this presentation, you'll find these equations delineated diversely. Consulting these references can furnish you with a more detailed grasp of the equation's essence.
The energy disbursed by the respiratory system during a single cycle of inhalation is designated as mechanical power. The terms mechanical power and energy delivered by the respiratory system during a single inspiration cycle are used interchangeably. This is a means of comprehensively portraying the mechanics underlying ventilator-induced lung injury (VILI), capturing its intricacies.
- Stress = Force applied to the lungs (Driving Pressure)
- Strain = Response to applied stress (Distortion)
- Frequency = number of cycles (rate)
- Mechanical Power is the relationship between stress, strain and frequency Joules/time
- If driving pressure of > 16 is injurious, then driving pressure of > 16 X 30 times a minute must be worse (more power applied to the lungs)
- Example = hopping on sprained ankle, once versus all day long
Mechanical Power: A New Concept in Mechanical Ventilation (Paudel et al. 2021)
Fast forward a few years to a couple of years ago, we encountered the emergence of a novel concept in mechanical ventilation (Paudel et al. 2021). To save time, I will not delve into the formulas any further. However, this recent paper essentially outlines our understanding of mechanical power and its constituent elements.
Let's delve deeper into the terminology we have previously mentioned. Stress, in literature, pertains to the actual force exerted on the lungs. Specifically, in this context, we refer to it as driving pressure. This driving pressure stands as the primary stress experienced during a respiratory cycle, defined as the difference between the lowest pressure (PEEP) and the highest pressure (plateau pressure) believed to be exerted on the alveoli.
Strain, on the other hand, represents the reaction to stress. When stress is applied, strain is the subsequent effect on the alveoli, akin to how each alveolus can bear varying degrees of strain. Frequency corresponds to the number of these cycles occurring within a minute—the respiratory rate. This could be either the rate administered by the ventilator or the patient's spontaneous breathing rate. Throughout the literature, you'll encounter descriptions of mechanical power as joules over time.
Mechanical power signifies the interconnectedness of stress, strain, and frequency. As an illustration, consider a patient with a driving pressure of 16, indicating injurious levels. If the rate of cycles increases beyond what's set, let's say 30 times a minute, it logically worsens the situation compared to a lower rate. This scenario should be averted as it results in heightened power applied to the lungs. To elucidate, think of someone repeatedly hopping on a sprained ankle—the analogy holds parallels to an injured lung. The constant, higher frequency of energy delivery exacerbates the situation.
A pertinent analogy involves bending a paperclip. Just as you apply stress to the paperclip by bending it repeatedly, the alveoli undergo a similar process. They experience stress from the cyclic opening and closing, leading to strain and injury. This mirrors the phenomenon of atelectrauma. Much like a sprained ankle, inflammatory responses are triggered—capillaries leak, cytokines are released, and an attempt to heal initiates, causing swelling. The alveoli, illustrated earlier, undergo comparable processes within the surrounding fluid environment.
Understanding these concepts aids in comprehending the interplay of stress, strain, and frequency within mechanical power—a comprehensive lens through which we examine ventilator-induced lung injury.
The Impact of Mechanical Power on Mortality in ARDS Patients in Intensive Care (Coppola et al., 2020)
The impact of mechanical power on mortality in ARDS patients in intensive care (Coppola et al., 2020) study presents intriguing findings. The key finding of this paper suggests that while the anticipation of mechanical power did not exert a significant clinical impact overall, there's an important distinction when comparing lungs with normal and abnormal compliance. Specifically, when applying the same energy to patients with these differing lung compliance levels, those with poorer compliance experienced worse outcomes.
Ventilator-related Causes of Lung Injury: The Mechanical Power (Gattinoni et al., 2016)
Another relevant study by Gattinoni, known for the PROSEVA trial and related literature on proning, provides another example of the mechanical power equation. This equation is essentially an advanced iteration of the equation of motion, incorporating factors such as respiratory rate, and more. You may find it valuable to explore these concepts in depth at a later time. The authors speculate that in the future, ventilators' software algorithms may evolve to report the energy applied to the lung, allowing for prompt reactions based on this information.
Reducing Mechanical Power
- Reduce the number of cycles
- Permissive hypercarbia
- Improve compliance
- Diuresis
- Treat infection
- Effusions
- Redistribute Stress
- Prone positioning
- PEEP
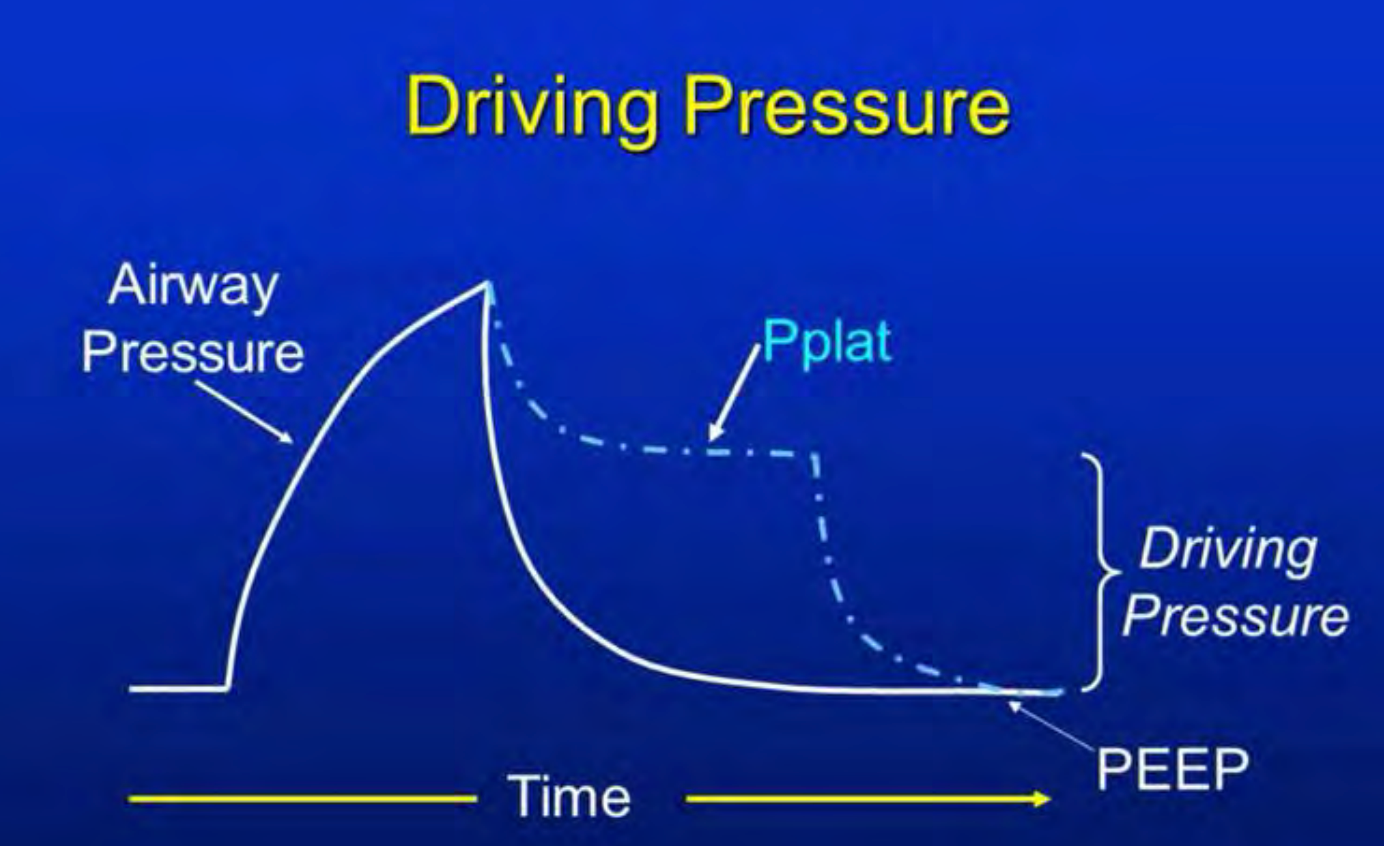
Figure 3. Scalar representation of driving pressure.
Figure 3 illustrates the graphical representation of mechanical power. I encourage you to take your time and familiarize yourself with it when you have the opportunity. Our ultimate goal is to reduce mechanical power. How can we achieve this objective? Let me share a recent patient encounter, though I will provide a concise overview due to time constraints. The trauma case patient required substantial pressure support due to shock and ongoing bleeding. Impressively, he received 90 units of blood products. His condition was characterized by shock and acidemia, resulting in a severe mixed acidosis with a pH below seven. His CO2 levels were also elevated in the 50s, even though his minute volume was as high as 18 liters per minute.
For a patient in such a challenging condition, the options available to mitigate mechanical power are inherently limited, given the intricacies of their case. This complexity underscores the necessity of a multifaceted approach. However, when dealing with a less intricate case, where the degree of acidemia is less pronounced, and the mixed acidosis is milder, the solution might be as straightforward as reducing the respiratory rate – embracing permissive hypercapnia. In this context, it is crucial to prioritize concern over the driving pressure and the quantum of energy being imparted to the lungs, rather than fixating solely on CO2 levels. This introduces a trade-off: if the choice lies between diminishing mechanical power and permitting a moderate CO2 elevation, a careful assessment of the patient is warranted to determine the risk-to-benefit ratio. As we navigate these considerations, one must not overlook avenues to enhance compliance.
Patients can potentially benefit from diuresis in cases where compromised compliance results from an infectious process or pleural effusions. These effusions can be drained if necessary to enhance compliance and minimize energy exerted on the lungs. Additionally, the concept of redistributing stress-strain ratios is pivotal. While an increase in pO2 might not always correlate with improved outcomes, it is plausible that this metric isn't the sole determinant. Take prone positioning as an example; it may not primarily elevate pO2, leading to better outcomes but rather the alteration in transpulmonary pressure. Reducing stress-strain levels could potentially correlate with lower mechanical power expenditure.
PROSEVA Trial: Prone positioning in severe acute respiratory distress syndrome (Guérin et al., 2013)
A valuable technique in enhancing compliance is Positive End-Expiratory Pressure (PEEP), which not only aids in alveolar recruitment but also curbs driving pressure. The PROSEVA study group (2013), a well-known landmark study in critical care, demonstrated the remarkable impact of proning. By proning patients with P/F ratios below 150 for 16-hour intervals, mortality rates were effectively halved.
Case Review
To illustrate the integration of these concepts, let's delve into a concise case study. A previously healthy 31-year-old male encountered a few days of illness, prompting his arrival at the Emergency Department due to fever and chills.
Upon assessment, he exhibited hypotension, tachypnea, and a somewhat elevated white cell count. Additionally, he was experiencing hypoxemia. A glimpse of his vital signs is presented here: marked hypotension, evident tachypnea, and hypoxemia even on room air. His white cell count showed a slight elevation, while other laboratory results remained within acceptable ranges. Notably, his primary acidemia was rooted in metabolic factors, highlighted by a base deficit of nine.
In response, the initial measure involved administering high-flow nasal cannula oxygen; however, this approach yielded no significant improvement. Eventually, the decision was made to proceed with intubation, utilizing an 8.0 oral endotracheal tube. Subsequently, a chest X-ray was conducted, and he was promptly transferred to the Intensive Care Unit. A central line was established, and normal saline infusion was initiated to address his hypotensive state.
The patient displayed significant ARDS, likely of viral origin. He was initiated on volume-assist controlled ventilation with a tidal volume set at six milliliters per kilogram of predictive body weight, amounting to approximately 380 milliliters. The respiratory rate was set at 10 breaths per minute, and the Positive End-Expiratory Pressure (PEEP) was maintained at 10. I have marked these values in red, highlighting what I consider to be the most potentially injurious aspects. Notably, he received 100% FiO2, yielding a plateau pressure of 36 and an abnormally elevated driving pressure of 26, signifying a significant concern.
Conducting a follow-up arterial blood gas analysis, we observe several changes in the patient's condition. His pCO2 has decreased, leading to a slightly improved pH level. However, his pO2 remains relatively unchanged, resulting in a P/F ratio of 67, which is indicative of severe ARDS. This raises a crucial question: What steps should we take next?
Our strategy revolves around addressing the driving pressure, especially since it was notably high at 26 immediately following intubation. Through strategic adjustments, such as reducing his tidal volume and increasing PEEP, we have successfully managed to lower his driving pressure to 16 – a significant improvement, albeit still somewhat elevated. Subsequent blood gas analysis reveals results that, while not ideal, are not causing undue concern.
While our goal is continuous improvement, our primary focus remains on preventing any adverse ventilator-induced effects. Our commitment to pursuing the correct course of action remains unwavering. Consequently, we maintained the same tidal volume while raising PEEP. His persistent high FiO2 requirement and elevated plateau pressure propelled this choice. However, it is noteworthy that the recruitment maneuvers led to a driving pressure below 15, signifying progress in this aspect.
Upon follow-up blood gas analysis, we observe positive trends in the patient's condition. His acidosis is improving, pO2 levels are increasing, and the P/F ratio is showing improvement as well. As a result of these positive changes, we are able to gradually decrease his FiO2 to 50%. Notably, his plateau pressure measures at 29. By subtracting the PEEP value, we calculate a driving pressure of nine, signifying a much safer condition. The application of PEEP for recruitment has played a crucial role in achieving this reduction in driving pressure and, consequently, mechanical power. This shift towards a safer state has also enabled us to lower the FiO2, further contributing to the reduction of driving pressure and overall mechanical power.
This case emphasizes the spectrum of solutions, which can range from simple to complex, as exemplified by the contrast between the patient mentioned earlier and the current case. In this particular scenario, PEEP recruitment has yielded positive outcomes. However, in scenarios where this approach may not prove as effective, alternative interventions could encompass reducing tidal volume while tolerating modest changes in pH, adjusting the respiratory rate with similar pH considerations, and implementing diuresis to maintain drier conditions for compliance, among other strategies. Furthermore, considering culture-specific antibiotics, when suitable, might enhance compliance and overall progress.
The concept of mechanical power revolves around the energy transmitted from a mechanical ventilator to the lungs during the breathing process. Acquiring a grasp of mechanical power offers valuable insights into the components of a mechanically-assisted breath that potentially contribute to ventilator-induced lung injury. Mitigating this energy burden on the lungs leads to improved patient outcomes. While reducing mechanical power is often straightforward, certain cases require a more intricate approach, demanding close collaboration among healthcare professionals and a multidisciplinary team. This collaborative effort involves exploring a range of strategies to optimize patient outcomes and minimize ventilator-induced complications.
References
Acute Respiratory Distress Syndrome Network, Brower, R. G., Matthay , M. A., Morris, A., Schoenfeld, D., Thompson, B. T., & Wheeler, A. (2000). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and th e acute respiratory distress syndrome. The New England journal of medicine, 342(18), 1301 – 1308.
Amato, M. B., Meade, M. O., Slutsky, A. S., Brochard , L., Costa, E. L., Schoenfeld, D. A., Stewart, T. E., Briel , M., Talmor, D., Mercat , A., Richard, J. C., Carvalho, C. R., & Brower, R. G. (2015). Driving pressure and survival in the acute respiratory distress syndrome. The New England journal of medicine, 372(8), 747 – 755.
Bellani , G., Laffey, J. G., Pham, T., Fan, E., & LUNG SAFE Investigators and the ESICM Trials Group (2016). The LUNG SAFE study: a presentation of the prevalence of ARDS according to the Berlin Definition!. Critical care (London, England), 20(1), 268.
Coppola, S., Caccioppola , A., Froio , S., Formenti , P., De Giorgis , V., Galanti , V., Consonni , D., & Chiumello , D. (2020). Effect of mechanical power on intensive care mortality in ARDS patients. Critical care (London, England), 24(1), 246.
Gattinoni , L., Tonetti , T., Cressoni , M., Cadringher , P., Herrmann, P., Moerer , O., Protti , A., Gotti , M., Chiurazzi , C., Carlesso , E., Chiumello , D., & Quintel , M. (2016). Ventilator - related causes of lung injury: the mechanical power. Intensive care medicine, 42(10), 1567 – 1575.
Guérin , C., Reignier , J., Richard, J. C., Beuret , P., Gacouin , A., Boulain , T., Mercier, E., Badet, M., Mercat , A., Baudin , O., Clavel , M., Chatellier , D., Jaber, S., Rosselli , S., Mancebo , J., Sirodot , M., Hilbert, G., Bengler , C., Richecoeur , J., Gainnier , M., … PROSEVA Study Group (2013). Prone positioning in severe acute respiratory distress syndrome. The New England journal of medicine, 368(23), 2159 – 2168.
Paudel , R., Trinkle, C. A., Waters, C. M., Robinson, L. E., Cassity , E., Sturgill, J. L., Broaddus, R., & Morris, P. E. (2021). Mechanical Power: A New Concept in Mechanical Ventilation. The American journal of the medical sciences, 362(6), 537 – 545.
Ware, L. B., & Matthay , M. A. (2000). The acute respiratory distress syndrome. The New England journal of medicine, 342(18), 1334–1349.
Citation
Lamb, K (2023). Mechanical power at a glance. Continued.com - Respiratory Therapy, Article 196. Available at www.continued.com/respiratory-therapy
