Editor’s note: This text-based course is an edited transcript of the webinar, Objective Measures of Asthma, presented by Kevin Collins, PhD, RRT, RPFT, AE-C.
It is recommended that you download the course handout to supplement this text format.
Learning Outcomes
After this course, participants will be able to:
- List the objective measures utilized in the diagnosis and management of asthma
- Define the terms asthma control and asthma severity
- Describe how lung function testing may aid in diagnosing and managing asthma
- Describe how the fractional concentration of exhaled nitric oxide may be used as part of an ongoing asthma monitoring and management strategy
- Describe how the measure of blood eosinophils may aid in diagnosing asthma
Asthma Facts in the U.S.
Some of you may already be familiar with the following information as respiratory therapists, but it is crucial to acknowledge the prevalence of asthma and its diagnostic challenges. In the United States alone, there are at least 25 million individuals with a confirmed asthma diagnosis, with approximately 20 million being adults over the age of 18 and around five million being children under 18 years old. Interestingly, as children with asthma mature, the gender distribution changes, resulting in more female adults being affected than male adults.
Objective Measures of Asthma
It is essential to recognize that asthma does not have a single objective measure for diagnosis. There is no single blood test or lung function test that can definitively identify the condition. Consequently, we face considerable challenges in the diagnostic process. Instead, we rely on various objective measures in conjunction with patients' medical history and physical examination to ascertain an accurate asthma diagnosis.
Measures utilized for diagnosing, monitoring, and management of asthma:
- Spirometry/Peak Flow Monitoring
- Bronchodilator Study (i.e., before - & after - spirometry)
- Fractional Concentration of Exhaled Nitric Oxide (FeNO)
- Complete Blood Count (CBC): Blood Eosinophils
- Bronchoprovocation Study (e.g., Methacholine Challenge)
- IgE Mediated Allergies (e.g., positive skin test)
Our focus will be on several specific measures employed for diagnosing, monitoring, and managing asthma. These include spirometry and peak flow monitoring, bronchodilator study, fractional concentration of exhaled nitric oxide (FeNO), complete blood count (CBC) with a particular emphasis on the differential count and, specifically, blood eosinophils, which play a significant role in asthma. Additionally, we will explore the bronchoprovocation study, also known as a methacholine challenge. This study is particularly useful when a patient exhibits signs and symptoms of asthma, but their spirometry results appear normal. In such cases, a physician may request a bronchoprovocation study, where the patient inhales increasing doses of methacholine to induce bronchospasm and assess the response. By utilizing these objective measures and carefully evaluating each patient's unique clinical presentation, we can improve the accuracy of asthma diagnosis and subsequently tailor effective management strategies.
Individuals with asthma experience a significant decrease in lung function values, specifically the Forced Vital Capacity (FVC) and Forced Expiratory Volume in one second (FEV1), often dropping by 20% or more during asthma episodes. Furthermore, asthma is often associated with IgE-mediated allergies. The allergic disease occurs when a person's immune system is triggered by allergens they inhale, such as pollen, pet dander, dust mites, chemicals, and strong odors. Diagnosis of allergic triggers can be made through a skin test known as a RAST (Radioallergosorbent test), where allergens are injected under the patient's skin to assess their sensitivity and identify the allergens that contribute to their allergic disease.
Assessment & Monitoring
- Asthma is a chronic inflammatory airways disease
- Asthma Control is defined as the degree to which the manifestations of asthma are minimized and the goals of therapy are met.
- Asthma Severity is defined as the intrinsic intensity of the disease process.
- Intermittent
- Mild Persistent
- Moderate Persistent
- Severe Persistent
Asthma is characterized as a chronic inflammatory airway disease with persistent inflammation even during non-episodic periods. Being a chronic disease means that there is no cure, and its unpredictability and potential debilitation pose significant challenges for management. Asthma control is defined by the degree to which asthma symptoms are minimized and treatment goals are achieved. As respiratory therapists, we are familiar with manifestations of asthma, such as wheezing, coughing, and shortness of breath, and the ultimate goal is to enable patients to lead a normal life by attending school, working, and engaging in physical activities without limitations.
Notably, persistent coughing, especially at night, can serve as a warning sign of an impending asthma episode. Monitoring and assessing asthma regularly is crucial, as it helps in the early detection of exacerbations and the implementation of appropriate management strategies to maintain optimal asthma control and improve patients' overall quality of life.
If patients are complaining of coughing, especially during nighttime, and they say it is interrupting their sleep, that is an ominous sign, and they need to see their physician to be checked out. Patients who complain of persistent coughing, especially at nighttime, interrupting their sleep, should take it as an ominous sign and promptly seek medical attention from their physician to get checked out. Asthma severity refers to the intrinsic intensity of the disease process. According to the American Thoracic Society, severe asthma is defined as when a patient requires maximum doses of inhaled corticosteroids and a second controller, such as a long-acting beta-agonist, for at least 50% of the year. While some individuals experience severe asthma throughout their lives, others, including adults, can develop asthma at any stage in life. Eosinophilic asthma, a specific type of asthma, will be discussed later in this presentation.
In national guidelines like EPR 3 or GINA, asthma is usually classified into four categories: intermittent, mild persistent, moderate persistent, and severe persistent. These classifications are based on the patient's symptoms, lung function tests, and other relevant parameters. Understanding asthma severity guides healthcare professionals in determining the appropriate treatment interventions for patients. Therefore, the medication regimen for someone with intermittent asthma will likely differ from that of a person with severe persistent asthma.
Asthma Control: Rules of Two
- Does the patient....
- Use a reliever inhaler more than TWO TIMES A WEEK?
- Awaken at night with asthma more than TWO TIMES A MONTH?
- Refills his/her reliever inhaler more than TWO TIMES A YEAR?
- Use prednisone TWO or more times a year for flares of asthma?
- Measure changes in PEF with asthma symptoms of more than TWO TIMES 10 (20%)?
- If the patient answers YES to any of these questions, he/she needs to consult with their doctor
- *Rules of Two is a registered trademark of the Baylor Healthcare System
A simplified approach called "Rules of Two" was developed by a pulmonary doctor at Baylor College of Medicine in Dallas. This approach helps patients assess if their asthma is under control. For instance, they may need to contact their doctor if they are using their reliever inhaler more than two times a week, excluding instances when they use it for pre-treating before exercise. Additionally, if they experience nighttime asthma-related awakenings more than two times a month, it warrants medical attention as well. Regularly monitoring asthma symptoms is essential for maintaining optimal control and taking appropriate action when needed. Refills his or her reliever inhaler more than two times a year.
Refilling the reliever inhaler more than twice a year can be telling. For instance, an albuterol metered-dose inhaler (MDI) contains 200 actuations or puffs. If a patient refills their inhaler three times a year, that amounts to 600 puffs, indicating potential overuse (more than twice a week). Similarly, if a person requires prednisone, an oral steroid, two or more times annually to manage asthma flares, it poses serious risks. Prednisone, a synthetic form of cortisol, our body's natural hormone for combating infection and inflammation, can effectively suppress inflammation during asthma episodes. However, given that cortisol is taken up by every cell in the body, the uptake of prednisone can cause numerous adverse effects. Frequent prednisone use is an indicator of uncontrolled asthma, putting individuals at risk of impairment, exacerbation, and even fatal asthma episodes.
Another concerning sign of uncontrolled asthma is when peak expiratory flow measurements, taken with asthma symptoms occurring more than twice, show a drop of 20% from the patient's personal best baseline. A decline of this magnitude warrants immediate medical attention. Patients who respond affirmatively to any of these indicators should consult their physician to ensure proper asthma control and an appropriate treatment regimen.
Spirometry/Peak Flow Monitoring
Spirometry
- The most readily available and useful pulmonary function test for the measure of flow rates and lung volumes.
- It is a key diagnostic test for asthma
- Measured values include FVC, FIVC, FEV1, FEV1/FVC% (i.e., the ratio), FIF50%, FEF50%, FEF25% - 75%, and PEF
- EPR - 3, national asthma guidelines recommends spirometry to be performed at least every 1 - 2 years; more frequently for asthma that is not well controlled
- American Thoracic Society (ATS) revised guidelines published in 2019 (www.thoracic.org)
- Example: Reference set recommended is GLI 2012
Let's delve into the first category - spirometry and peak flow monitoring, examining their respective differences and applications in asthma management. The first category we will explore is spirometry and peak flow monitoring, highlighting the differences between these two methods.
Spirometry is the most readily available and valuable pulmonary function test for measuring flow rates and lung volumes. It is a straightforward test to conduct and serves as a key diagnostic tool for asthma. Spirometry is considered the gold standard test for determining airway obstruction, which is a characteristic feature of asthma.
During spirometry, several measured values are obtained, including Forced Vital Capacity (FVC), Forced Inspiratory Vital Capacity (FIVC), Forced Expiratory Volume at one second (FEV1), FEV1/FVC percent ratio, Forced Inspiratory Flow Rate at 50% (FIF50%), Forced Expiratory Flow Rate at 50% (FEF50%), Forced Expiratory Flow Rate at 25 to 75% (FEF25-75%), and Peak Expiratory Flow Rate (PEFR). The specific variables included in the spirometry report may vary depending on the spirometer's format, and it's essential to consult with the medical director or the overseeing physician of the PFT (Pulmonary Function Testing) lab to determine the appropriate reporting format.
According to the national asthma guideline, individuals diagnosed with asthma should undergo spirometry once or twice a year, as ordered by their physician. For those with uncontrolled asthma, more frequent spirometry may be necessary. I recommend visiting the American Thoracic Society website, as they are the group responsible for writing guidelines and recommendations on spirometry and other lung function testing. They updated the spirometry guidelines in 2019, which is essential to follow if you are responsible for performing spirometry in your facility or area of practice as a respiratory therapist, whether in-home care, rehabilitation, or elsewhere.
For example, one change from the previous guidelines in 2005 is the recommended reference set by the American Thoracic Society, known as the Global Lungs Initiative (GLI) 2012. This reference set establishes normal values for individuals undergoing spirometry. Ensuring compliance with these latest guidelines and using an updated spirometer is vital for accurate and reliable test results. It is crucial to have the correct recommended reference set in the spirometer device. When purchasing a spirometer, it contains numerous reference sets, and one must scroll through the list to find the GLI 2012 reference, which is the recommended set for spirometry in the U.S., Europe, Asia, and Canada. Ensuring the use of the appropriate reference set is essential for accurate and standardized spirometry results.
- Used to aid in the diagnosis of asthma because . . .
- Spirometry provides more precise information
- Spirometers are easily calibrated
- Results are accompanied by predicted values for each patient
- Examination of the volume - time & flow - volume curves enables assessment of patient effort
- It is the gold standard test to diagnose airways obstruction
Using spirometry in the diagnosis of asthma offers several advantages, particularly in comparison to peak flow monitoring. Spirometers are easily calibrated, and it is recommended to calibrate the device using a three-liter syringe before conducting testing. However, some handheld spirometers have technology that eliminates the need for calibration before each test. It is essential to verify the type of spirometer being used and whether the manufacturer requires calibration with a three-liter syringe before testing.
Apart from calibrating the spirometer, it is equally important to calibrate the three-liter syringe annually. Typically, this calibration service is provided by companies such as the Hans Rudolph Company, which checks and ensures the accuracy of the three-liter syringe by verifying that it correctly measures three liters. By utilizing spirometry with the proper reference set and ensuring regular calibration, healthcare professionals can obtain precise and reliable information for asthma diagnosis and monitoring, improving patient care and management.
Another significant advantage of using a spirometer in diagnosing asthma is that the results come with predicted values for each patient derived from the selected reference set, such as the GLI 2012. These predicted values establish what is considered normal lung volumes and flow rates for an individual based on factors like age, measured height, weight, race, ethnicity, and sex at birth. By comparing an individual's actual measured values to their predicted values, we can determine the percentage of normal for that specific individual, accounting for factors that influence spirometry results.
Spirometry offers further precision by allowing us to examine the flow-volume time curve and flow-volume loop, enabling the assessment of patient effort. It is crucial for respiratory therapists to be skilled in interpreting these graphical representations, understanding what normal should look like, and accurately judging a patient's effort during the test.
Moreover, spirometry serves as the gold standard test for diagnosing airway obstruction, a characteristic feature of asthma. However, it may also hint at the presence of airway-restrictive disease, although lung volumes need to be performed to confirm this. A reduced vital capacity in spirometry results can suggest the possibility of restrictive disease.
- Spirometry is used to identify the characteristic features of asthma, which include:
- Variable airflow limitation, which can be either daily or episodic in nature
- Airflow limitation that reverses with bronchodilator administration
- Airways hyperresponsiveness, which is an excessive decrease in airflow in response to specific stimuli or "triggers" (e.g., pollen, animal dander, dust mites, mold)
Spirometry is particularly valuable in identifying the variable airflow limitation that is characteristic of asthma, which can manifest either daily or episodically. The unpredictable nature of asthma means that variable airflow limitation can occur at any time, and spirometry helps in detecting and monitoring these fluctuations. It could be unpredictable and come up at any time, depending on if they have allergic disease and are exposed to a potential trigger for that individual. Airflow limitation that reverses with a bronchodilator administration. In adult patients, we aim to determine if they have pure asthma or a combination of emphysema or chronic bronchitis, especially if they are chronic cigarette smokers. Another consideration is airway hyperresponsiveness, where the airways show excessive constriction in response to triggers like grass or tree pollen, animal dander, dust mites, or mold.
To detect airflow limitation in patients with asthma, several methods can be used, including spirometry before and after bronchodilator administration, peak expiratory flow measurement, and analysis of flow volume loops produced by the Forced Vital Capacity (FVC) or Forced Inspiratory Vital Capacity (FIVC) maneuver. Each of these approaches provides valuable insights into a patient's lung function and aids in the diagnosis of asthma.
Spirometry: Before & After Bronchodilator
Spirometry before and after bronchodilator is the preferred method for diagnosing asthma because it offers higher precision and can be calibrated. Additionally, using predicted values allows for a more accurate assessment. Analyzing the flow volume loops helps evaluate patient effort and bronchospasm reversibility, which is essential in determining the presence of airway obstruction. The ratio of Forced Expiratory Volume in one second (FEV1) to Forced Vital Capacity (FVC) is also examined during this test. Peak expiratory flow monitoring is another method used to assess airflow limitation. However, spirometry is generally considered more reliable due to its precision and ability to provide more comprehensive data. By utilizing these diagnostic methods, healthcare professionals can effectively evaluate and diagnose asthma, enabling the implementation of appropriate treatment plans to manage the condition and improve patients' respiratory health.
In adult patients, it is essential to differentiate pure asthma from asthma and COPD overlap, especially in chronic smokers. For non-smoking adults without a high suspicion of emphysema or chronic bronchitis, we can assess if their airway obstruction is reversible using a bronchodilator. To determine the need for bronchodilator studies, we examine the FEV1 to FVC percent ratio, which measures the amount of air a person can exhale in the first second (FEV1) compared to the total amount of air exhaled (FVC). A normal FEV1/FVC ratio is around 75 to 80%. If this ratio is less than predicted, it indicates possible airflow obstruction, which could result from inflammation, excess mucus, or bronchospasm. To confirm the presence of reversible airflow obstruction, we administer a bronchodilator, such as albuterol, and wait for 15 minutes, the peak onset of action for short-acting beta agonists like albuterol. Waiting for the peak effect ensures precise and valid measurement of lung function.
An anecdotal example highlights the importance of waiting for the peak effect of the bronchodilator. In a case involving my three-and-a-half-year-old son, the nurse practitioner administered a Xopenex nebulizer treatment and assessed his lung sounds immediately after the treatment. However, my son's airways were still constricted at that moment, and the wheezing only became audible 10 minutes later when the bronchodilator's peak effect took effect. In summary, accurately evaluating reversible airflow obstruction using spirometry and bronchodilator studies aids in diagnosing asthma and providing appropriate management for patients, especially those without underlying COPD components or chronic smoking history. I posed the question to my students, "What happened?" The reason for the nurse practitioner's failure to hear anything, not to criticize her profession, but to inquire, was due to her lack of waiting for the peak effect of the short-acting beta agonist. Consequently, my son's airways were so constricted that he could not breathe enough air to produce a wheeze until the bronchodilator took effect.
The 15-minute wait time after administering the bronchodilator is crucial to observe its peak effect. According to the ATS criteria, significant reversibility of airway obstruction is determined by increases greater than 12% and 200 milliliters in either FVC or FEV1. In the provided spirometry report, we focus on the FVC and FEV1 values. The FEV1 shows a change of more than 12%, meeting the criterion. However, the FVC doesn't meet the 12% change requirement. To check for the 200-milliliter increase, we compare the pre-measured value of 1.34 liters with the post-measured value of 1.57 liters. The difference is indeed greater than 200 milliliters, indicating a significant bronchodilator response in this patient. Therefore, the pulmonary doctor would include in the interpretation that the patient exhibited a significant response to the bronchodilator. To back up a minute, how do we know that they have airway obstruction? Well, their predicted ratio of FEV1/FVC is 78, and this patient's measured is 63. In summary, they definitely have airway obstruction.
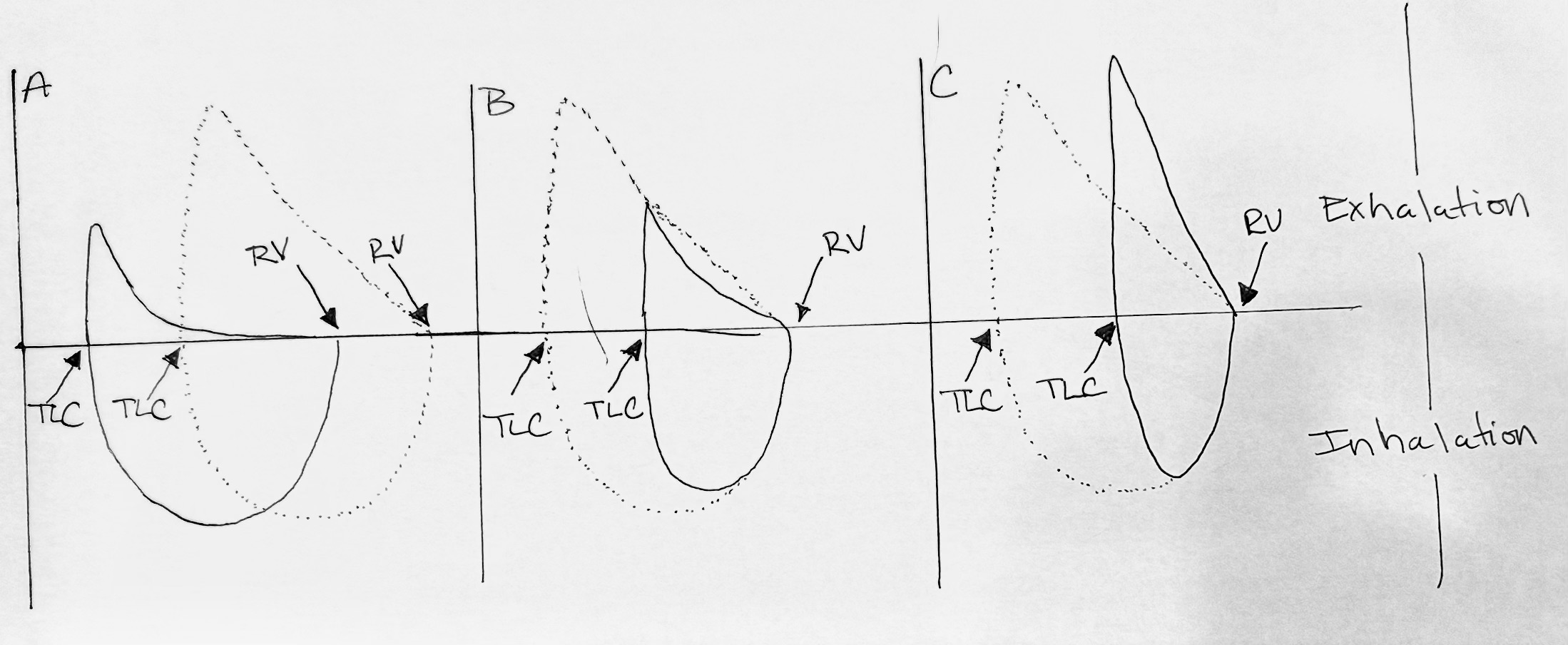
Spirometry: Flow-Volume Loop
Figure 1. Flow-volume loop and volume time graph of pulmonary function test.
- (A) Airflow limitation caused by inflammation, excess mucous, & bronchospasm
- (B) Reduced airflow limitation and reduced lung volumes
- (C) Reduced lung volumes and increased flow
The flow volume loop in Figure 1, holds significant importance in distinguishing normal and abnormal respiratory patterns. Hence, three distinct flow volume loops can be identified in Figure 1. The first, loop A, demonstrates airflow limitation, while loop B indicates mixed disease and loop C reveals a restrictive component.
The airflow limitation is characterized by a classic concave appearance on the expiratory side of the flow volume loop, positioned above the horizontal line. When observing loop A, the expiratory side of the loop, located above the mentioned horizontal line, exhibits a concave shape.
The dotted lines in the flow volume loop represent a "normal" pattern, although they do not pertain to a real patient. In contrast, a mixed disease (loop B) would exhibit a reduced forced vital capacity and airflow limitation. On the other hand, a restrictive disease (loop C) would display reduced volume while maintaining a normal flow rate. The flow rate is represented on the vertical axis, while the volume is on the horizontal axis. Even without numerical values, a graphical analysis allows us to observe that the flow rate of the restrictive disease is higher than that of loops A and B. Additionally, the volume for the restrictive disease appears to be less, with a tall and skinny curve characteristic of a classic restrictive pattern.
Spirometry Errors
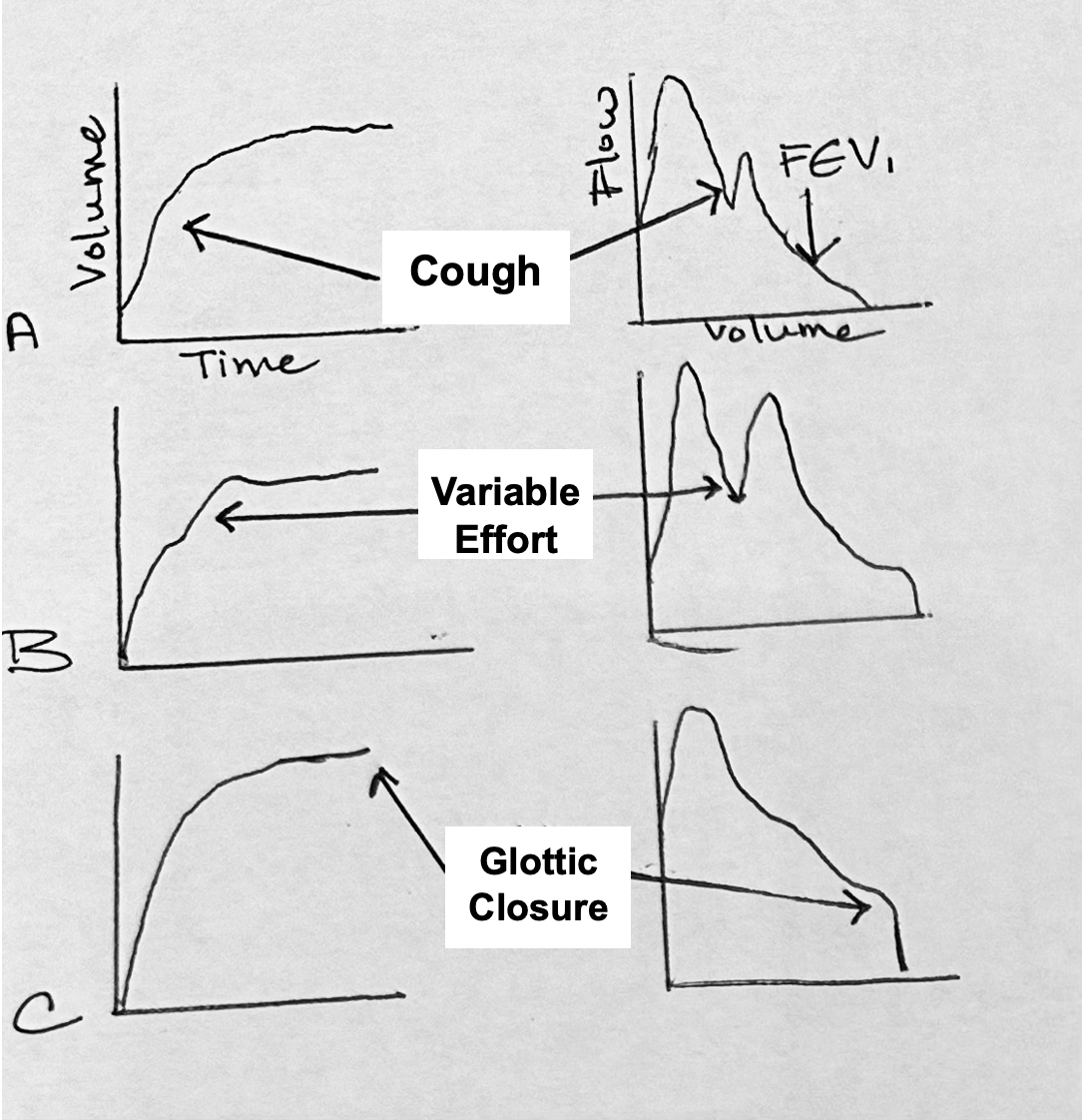
Figure 2. Volume and flow graphs illustrating spirometry efforts.
During the performance of spirometry, it is beneficial to have real-time feedback to identify patient efforts or errors accurately as seen in Figure 2. Certain errors may warrant the exclusion of data from interpretation. For instance, coughing during the first second can skew the ratio and would require the patient to perform the test again without coughing. Any coughing that occurs after the first second can be disregarded and the data can be deleted.
Variable effort occurs when a patient exhales, then inhales again before exhaling forcefully. To achieve reliable results, patients should take one smooth breath in, then exhale forcefully in one continuous breath before inhaling again.
The curves shown in the flow volume loop only represent the expiratory side, while the volume-time curve is depicted on the left. Glottic closure, though infrequent, can occur during spirometry. It happens when a patient closes off their vocal cords mid-effort, similar to performing a Valsalva maneuver. During glottic closure, there will be no flow, resulting in a straight line on the spirometry graph, similar to the pattern observed in ventilators.
Detection of Airflow Limitation
Peak Expiratory Flow (PEF)
Detection of airflow is facilitated through the utilization of peak flow measurements. Peak flow meters are widely prevalent and economically accessible to patients. Nevertheless, they cannot undergo calibration due to mass production. The depicted flow meter exemplifies a typical design suitable for both children and adults owing to its tapered structure. Conversely, certain meters are explicitly tailored for pediatric use, limited to a maximum capacity of 400 liters per minute, whereas adult variants typically reach 800 liters per minute. Patients can employ these meters to evaluate their symptoms and gauge their severity. The guidelines recommend the utilization of peak flow meters as an objective measure for patients who are unsure about their respiratory status, thus contributing to improved assessment accuracy.
It is more beneficial than waiting until their symptoms significantly worsen. By documenting their lung function using peak flow measurements, patients can potentially anticipate an impending asthma attack and seek treatment beforehand. Regular monitoring enables them to determine the severity of the attack and assess the efficacy of the treatment plan prescribed by their physician.
Another approach involves administering a peak flow meter to patients with normal spirometry before conducting bronchoprovocation testing. Patients would be asked to measure their peak flow twice or thrice in the morning and evening over a two to three-week period while their asthma is well-controlled and they are on maximum medication. The objective is to establish their personal best peak flow, which differs from the estimate provided on the nomogram that comes with the peak flow meter container. By knowing their personal best, they can effectively monitor their condition, using it as a reference point rather than relying on an estimated peak flow from the nomogram. During a maximal expiration effort from total lung capacity, the peak flow typically occurs within the first 200 milliseconds. This information is crucial for accurately interpreting peak flow measurements.
This indicates that patients must initiate the peak flow measurement with a forceful exhalation right from the start, taking a deep breath beforehand. The objective is to ensure that the peak flow measure does not vary by more than 40 liters per minute. For instance, if a patient's first effort results in a peak flow measurement of 400 liters per minute, and their second effort shows 325 liters per minute, the variability is too high. In such cases, we would encourage them to perform a third effort. If the third measurement, for instance, is 370 liters per minute, it would meet the acceptable criteria.
It is crucial to minimize variability in the patient's effort during peak flow measurement. Remember that peak flow meters report their measurements in liters per minute. In contrast, some spirometers can be configured to show measurements in liters per minute, while others may display results in liters per second. Be aware of this difference in units of measure, as the numbers will be significantly lower in liters per second than in liters per minute. When performing peak flow measurements on patients or instructing them to do so at home, advise them to record the highest of the three efforts in their diary. This process will help establish their personal best peak flow, as mentioned earlier.
Their personal best peak flow measurement can then be incorporated into their asthma action plan—a written document devised by physicians to determine the appropriate treatment regimen based on their peak flow readings. For instance, if their peak flow falls within the green zone, which constitutes 80 to 100% of their best personal peak flow, they are considered to be in good condition. However, if their peak flow drops into the yellow zone, usually ranging from 50 to 80% of their personal best, the physician may have prescribed instructions such as using their short-acting beta-agonist, the albuterol inhaler, by taking two puffs and waiting for 15 minutes before performing the peak flow measurement again, with the aim of elevating it back to the green zone. In contrast, if their peak flow falls into the red zone, which signifies less than 50% of their personal best, they are advised to contact their physician or, if instructed, proceed directly to the emergency room for evaluation. Thus, asthma action plans hold immense significance, and there are many individuals, both children and adults, who could potentially benefit from having one but are currently not utilizing it.
Complete Blood Count (CBC)
Blood Basics
Let's now proceed to discuss the complete blood count (CBC) and its correlation with eosinophils and asthma. As a brief overview, the four primary components of blood consist of plasma, red blood cells, also referred to as erythrocytes, and within red blood cells, we find hemoglobin, and hematocrit. Hemoglobin and hematocrit values can be obtained using an ABG analyzer, particularly if processed through the CoOX side, or they can be acquired from the CBC report issued by the main laboratory.
Components of WBC Count
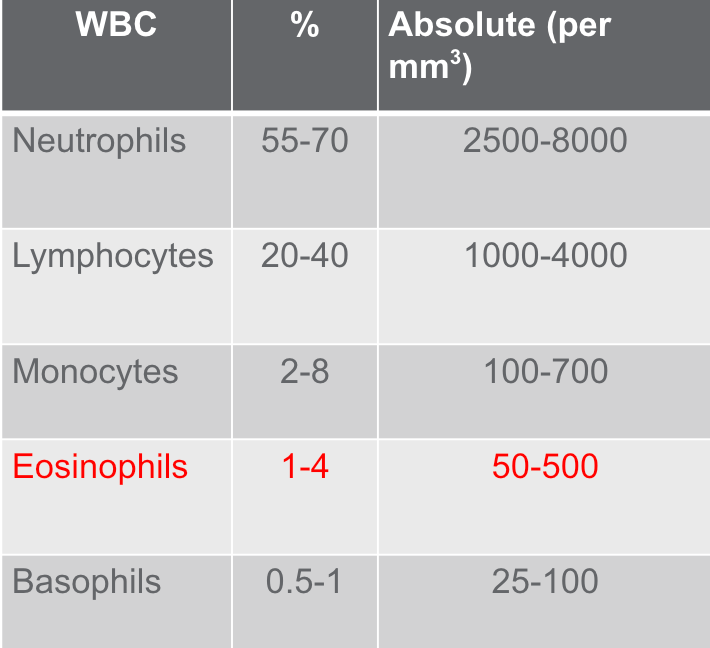
Figure 3. Components of WBC Count (Mosby’s Diagnostic & Laboratory Test Reference. Elseveir. St. Louis, MO)
Let's delve into a detailed examination of white blood cells (leukocytes) and platelets in Figure 3. The CBC is a fundamental test that is typically conducted on almost every hospitalized patient, as it provides critical insights into the types and quantities of cells circulating in the blood. The results of this test can aid in diagnosing various conditions, such as anemia, infections, and other disorders.
However, when it comes to patients with asthma, focusing on the differential count becomes particularly relevant. The differential count allows us to assess the various types of leukocytes and their percentages in one cubic millimeter of peripheral blood. It is good practice to take a moment and review the differential count, especially for patients with asthma, when analyzing a CBC report in settings like acute care, long-term acute care (LTAC), or outpatient facilities. By examining the percentages of each leukocyte type—neutrophils, lymphocytes, basophils, monocytes, and eosinophils—we can gather crucial information.
In the table provided, we can observe the percentage of white blood cells in relation to the total white blood cell count and the corresponding absolute measured value. This data can be of great significance in understanding the patient's immune response and may help evaluate their asthma condition more comprehensively.
Eosinophils
Figure 3 highlights eosinophils (Figure 3). Thus, our focus will be directed toward this component. Notably, eosinophils constitute a small percentage of total white blood cells, ranging from one to 4%, with an absolute count of 50 to 500. Our examination will center on eosinophils and their association with asthma. Despite significant research and technological advancements, the full extent of eosinophil's physiological functions remains incompletely understood.
Nevertheless, we can measure eosinophils in peripheral blood, as evident from the chart. However, it is essential to bear in mind that eosinophils primarily reside in tissues. In fact, the number of eosinophils in tissues surpasses the quantity found in free-circulating peripheral blood by several hundred folds. Healthy individuals typically have eosinophils distributed throughout various anatomical locations, including the airways, spleen, lymph nodes, thymus, and digestive tract.
Eosinophilia
Regarding eosinophils in relation to asthma, our primary interest lies in cell types that prompt the recruitment of eosinophils to tissues. This recruitment process is mainly instigated by cytokines—several types of cytokines will be discussed, as they stimulate these cells and initiate cell migration. Consequently, a cascade of events unfolds, ultimately culminating in airway inflammation and other related effects.
Eosinophilia is defined as a count of more than 500 eosinophils per microliter of blood in adults. Activated eosinophils can potentially cause tissue damage by releasing cytokines like transforming growth factors and interleukins (IL). In the context of asthma, specifically eosinophilic asthma, there is considerable focus on cytokines such as IL4, IL5, and IL13, which are widely discussed in the literature.
It is important to consider that these discussions revolve around the cellular pathways within the normal immune system. When interleukins (ILs) are activated and subsequently stimulate eosinophils, it can lead to tissue remodeling and fibrosis. This highlights the criticality of maintaining asthma control over time. In patients with eosinophilic asthma, repeated asthma exacerbations and eosinophil activation may cause long-term consequences, transforming their airways from a reversible asthma condition to a fixed airway disease resembling emphysema. Consequently, it becomes vital for such patients to be vigilant in managing their asthma to avoid these potential complications.
Asthma-induced eosinophilia can be differentiated from other causes of eosinophilia that may manifest with bronchospasm, mimicking asthma. For instance, helminthic infections (parasitic infections) or fungal infections may present similar symptoms. Therefore, if a physician identifies eosinophilic asthma in an adult with newly onset asthma, they will test for parasitic and fungal infections to ensure these are not the underlying causes of the bronchospasm. This precautionary step is crucial before initiating asthma therapy and treatment for the patient.
Eosinophilic Asthma
- A common type of severe asthma driven by an increased number of eosinophils.
- Increased eosinophils causes airway inflammation that can lead to asthma symptoms.
- Dyspnea, wheezing, fever, and blood-tinged sputum containing eosinophil-derived Charcot-Leyden crystals may also be present.
Eosinophilic asthma is a prevalent form of severe asthma characterized by an elevated eosinophil count. When these eosinophils become activated, bearing in mind their primary presence in tissues, they induce airway remodeling and inflammation. Consequently, the greater the number of activated eosinophils, the more pronounced the airway inflammation, leading to typical asthma symptoms such as coughing, wheezing, and shortness of breath.
To diagnose eosinophilic asthma in a patient with asthma, a sputum sample may be collected and sent to the lab. In eosinophilic asthma, the sputum sample may appear blood-tinged and contain crystals that are indicative of the eosinophil-driven nature of the condition, which can be observed under a microscope. This additional information aids in confirming the presence of eosinophilic asthma.
Asthma associated with eosinophilia is typically categorized as mild to moderate, with eosinophils in the blood measuring less than 1,500 microliters. However, if a patient's eosinophil count exceeds 1,500 microliters upon blood analysis, it should raise concerns about other eosinophilic pulmonary conditions, such as allergic bronchopulmonary aspergillosis. This possibility should be taken into account.
Eosinophils not only contribute to inflammation and asthma but can also affect other allergic disorders, including allergic rhinitis and atopic dermatitis. All these conditions are linked to an increased number of eosinophils. Consequently, considering eosinophil levels in a complete blood count (CBC) is vital. In the case of individuals with hypereosinophilic adult-onset asthma, the severity of their condition may require systemic glucocorticosteroids to control inflammation, particularly in the early stages of the disease.
Research indicates that hypereosinophilic adult-onset asthma is often associated with severe sinus disease, nasal polyposis, and, in some cases, aspirin-exacerbated respiratory disease. This emphasizes the importance of considering comorbidities when treating asthma. Addressing these additional conditions is essential, as asthma should not be viewed as a standalone disease. For patients with eosinophilic asthma, assessing and managing comorbidities like severe sinus disease, GERD, nasal polyps, and aspirin sensitivity become crucial aspects of their overall care.
Fractional Exhaled Nitric Oxide (FeNO)
- Nitric oxide (NO) is a gaseous molecule which can be detected in exhaled gas from the lower airways as the fraction of exhaled nitric oxide (FENO).
- FENO varies in health and disease; the exhaled air of asthmatic individuals contains higher levels of nitric oxide than healthy individuals.
- Studies reveal that exhaled nitric oxide levels rise in association with acute airway inflammation.
As we move forward, fractional exhaled nitric oxide (FeNO) becomes an important diagnostic test that we, as respiratory therapists, should be aware of. When we engage in the process of ventilation through exhaled gas, we inevitably exhale some amount of nitric oxide due to cellular metabolism. Thus, during the normal metabolism process, our cells produce nitric oxide, which can be measured when we exhale through this device.
FeNO levels vary in health and disease. Asthmatic individuals exhale higher levels of nitric oxide than healthy individuals. Consequently, research has concluded that the elevated FeNO levels in the airways of asthma patients must be related to certain factors.
Research indicates that exhaled nitric oxide levels increase in association with acute airway inflammation. This makes it a valuable marker for airway inflammation, which cannot be detected or observed through other means. As a non-invasive test, it is especially suitable for both children and adults, as it only requires tidal volume breathing. Even small children, possibly as young as three or four years old, depending on their coordination, can easily perform the test.
One noteworthy aspect of nitric oxide measuring devices is that they will not provide an invalid measure. Correct tidal volume breathing is necessary to obtain a value from the device; otherwise, it will not yield any result. If a value is obtained, you can rely on it as a valid and precise measurement. The measurement is carried out using a chemiluminescence analyzer and is expressed in parts per billion. As per the national guidelines, the Expert Panel on Asthma conditionally recommends incorporating FeNO measurement as part of an ongoing asthma monitoring and management strategy, which includes frequent assessments. FeNO measuring devices can typically be found in allergists' offices and pulmonary physicians' practices. Additionally, some primary care physicians who manage asthma are also adopting FeNO devices due to their simplicity of use.
Interpreting the results of a FeNO measurement:
When interpreting the results, you will receive a single numerical value without any graphical representation in a FeNO measurement. A FeNO measurement below 25 parts per billion in adults and below 20 parts per billion in children under 12 suggests non-eosinophilic airway inflammation or the absence of airway inflammation. Conversely, a FeNO measurement exceeding 15 in adults or 35 in children indicates eosinophilic airway inflammation. FeNO values between 25 and 50 parts per billion in adults should be interpreted cautiously in light of the clinical situation. It is essential to recognize that there is no single perfect measuring device for acute inflammation in asthmatics. However, FeNO measurement serves as an additional tool to help monitor the inflammation of our patients with asthma.
Summary
In summary, spirometry plays a crucial role in diagnosing and monitoring asthma, serving as a key lung function test. As I mentioned earlier, spirometry serves as the gold standard test for determining airway obstruction. Additionally, we can incorporate a before and after bronchodilator study with spirometry. To do this, perform spirometry at least three times, ensuring that we adhere to the ATS good guideline efforts to achieve a valid and precise test. Following the initial spirometry, we can administer the bronchodilator, wait for 15 minutes, and then conduct another test. This process provides valuable information about the reversibility of the disease and offers more insights than just peak flow monitoring alone.
Furthermore, we should not underestimate the significance of the graphics provided by the flow volume loop and the volume-time curve. If you have a spirometer, whether it's a handheld device or not, it should be capable of producing both the flow volume loop and volume-time curve on the final report, along with the accompanying text data for analysis.
Therefore, the before and after bronchodilator study requires that we ask the individual to withhold their bronchodilators if they are on medication. It is essential for those responsible for scheduling spirometry tests at your facility, particularly in an acute care setting with outpatients, to provide accurate information to patients about withholding their medications. However, we must also emphasize that if the patient experiences significant distress and shortness of breath, they can use their short-acting beta agonist, such as albuterol.
Nevertheless, we aim for patients to refrain from using bronchodilators, allowing us to evaluate their lung function without medication interference. The duration of withholding bronchodilators varies depending on whether they are short-acting, long-acting, or super ultra-long-acting bronchodilators. It may range from four to six hours up to three days, depending on the specific medication, to ensure a comprehensive before and after bronchodilator study that accurately determines the reversibility of airway obstruction.
Now, let's discuss peak flow. Peak expiratory flow is a valuable tool to monitor asthma and document airflow variability in individuals with normal spirometry. If we have diagnosed a patient with asthma and are confident in their condition, they can use a peak flow meter once they establish their personal best. For the most accurate assessment, it is advisable to use the peak flow meter in conjunction with an asthma action plan.
However, it provides them with an objective measure to track their lung function over time and monitor the severity of asthma attacks. Based on their asthma action plan, it can guide them to either consult with their physician or seek emergency care, depending on the results of their peak flow measurement.
Another application of peak flow measurement, as discussed in the lecture, involves patients who visit the PFT (Pulmonary Function Test) lab with normal spirometry but complain of asthma symptoms such as shortness of breath, wheezing, and coughing. In such cases, we recommend providing them with a peak flow meter and monitoring them over a period of three to four weeks. The goal is to capture any variability in airflow.
It is essential to note that the lung function of asthma patients should be normal or near normal outside of an asthma episode due to the reversible nature of the disease. However, patients with severe persistent asthma or severe eosinophilic asthma may exhibit airway obstruction on spirometry, although they represent a small percentage of cases. Generally, a peak flow meter can be used to measure lung function.
Despite its usefulness, some physicians may be hesitant to provide patients with a peak flow meter, fearing that patients may not make good efforts, leading to unreliable data in the patient's peak flow diary. I can share my own experience with my 19-year-old son that when he was younger, he was quite competitive. He thoroughly enjoyed doing the peak flow measurements, and I would guide him to ensure that he placed his teeth and lips correctly on the mouthpiece. He would take a deep breath in and forcefully exhale the air. Even young children can perform valid peak flows. However, monitoring them over several weeks and observing their technique multiple times is necessary to ensure accuracy. Nevertheless, I believe that most children and adults can perform peak flow measurements consistently, allowing us to use it as a reliable tool for monitoring their asthma.
During a four-week monitoring period, we may observe airflow limitation indicated by peak flow readings. In such cases, the physician may proceed with further testing to potentially diagnose asthma. Moving on to the CBC and the differential count, the latter includes eosinophils. Elevated eosinophil levels can cause airway inflammation, leading to asthma symptoms. This information is particularly relevant for patients with adult-onset asthma, as it may indicate eosinophilic asthma. Eosinophils are primarily tissue-dwelling cells found in the peripheral blood, and their count can be accurately measured. When activated by cytokines such as interleukins (IL4, IL5, and IL13), eosinophils trigger a cascade of inflammation in the airways, resulting in asthma symptoms.
It is a valuable test to rule out eosinophilia, which can aid in excluding the possibility of eosinophilic asthma and the associated problems. The final topic discussed was fractional exhaled nitric oxide (FeNO), a non-invasive test that requires only tidal volume breathing. The patient needs to maintain a certain flow rate during the test. However, all devices come with an incentive screen to guide and encourage proper test execution. The screen displays a cartoon image that helps ensure correct performance.
As mentioned earlier, both small children and adults can easily undergo this test, which takes only a couple of minutes to complete. FeNO testing is conducted using a small desktop device that occupies minimal space. It serves as a surrogate measure for acute inflammation in the airways. Physicians may utilize it to adjust inhaled corticosteroid dosages, potentially stepping up to the next level or higher dosage. Overall, it proves to be a valuable tool in the comprehensive diagnosis and management of asthma over the long term.
In conclusion, I am delighted that you were present, and I appreciate your attention throughout the discussion. I hope you have gained new insights into asthma and its objective measures.
References
Almeshari, M. A., Stockley, J., & Sapey, E. (2021). The diagnosis of asthma: Can physiological tests of small airways function help? Chron Respir Dis, 18. doi: 10.1177/14799731211053332.
Dweik, R. A. (2022). Exhaled nitric oxide analysis and applications. Retrieved from www.uptodate.com.
García-Marcos, L., et al. (2023). Asthma management and control in children, adolescents, and adults in 25 countries: a Global Asthma Network Phase I cross-sectional study. Lancet Glob Health. doi: 10.1016/S2214-109X(22)00506-X.
Irvin, C. et al. Pulmonary function testing in asthma. Retrieved from www.uptodate.com.
Lommatzsch, M., et al. (2023). A2BCD: a concise guide for asthma management. Lancet Respir Med. Published Online. January 27, 2023. doi: 10.1016/S2213-2600(22)00490-8.
McCormack, M. C., Stoller, J. D., & Hollingsworth, H. (2020). Overview of pulmonary function testing in adults. UpToDate Clinical Resources Database. Retrieved from www.uptodate.com.
Mottram, C. D. (2017). Ruppel’s Manual of Pulmonary Function Testing. Elsevier, St. Louis, MO.
Pagana, T. N., Pagana, T. J., & Pagana, K. D. (2023). Mosby’s Diagnostic & Laboratory Test Reference. Elseveir. St. Louis, MO.
Weller, P. F., & Klion, A. D. Eosinophil biology and causes of eosinophilia. Retrieved from www.uptodate.com.
Citation
Collins, K. (2023). Objective Measures of Asthma. Continued.com - Respiratory Therapy, Article 188. Available at www.continued.com/respiratory-therapy