Editor’s note: This text-based course is an edited transcript of the webinar, What’s Anesthesia Got To Do With It, presented by Steven Vela, MSNA, CRNA.
Learning Outcomes
After this course, participants will be able to:
- Recognize the role of the anesthetist in the hospital setting
- Identify airway equipment and strategies for intubation
- Determine induction agents as a tool for optimization of intubation
- Describe the differences in sedation strategies for the ventilated patient.
- Recognize the function of neuromuscular blocking agents
- Explain the importance of the cuff leak during planned extubation
Introduction
Nurse anesthetists, the first health providers dedicated to the specialty of anesthesia, have their roots in the 1800s when nurses first gave anesthesia to wounded soldiers on the civil war battlefields. Today, certified registered nurse anesthetists (CRNAs) are advanced practice registered nurses with graduate-level education who enjoy two high degrees of autonomy. CRNAs provide anesthetics to patients in every practice setting and for every type of surgery or procedure. They are the sole anesthesia providers in nearly all rural hospitals and the primary anesthesia provider to the men and women in the US armed forces. On average days, we might be on code pager duty, meaning we respond to hospital-wide airway emergencies, code blues, or consult for airway management. Sometimes, we are consulted for planned extubations with patients who are at higher risk for post-extubation stridor in intensive care units. We are in the operating rooms providing anesthesia for various surgeries or assigned to the obstetrician-gynecologist department where regional anesthesia is administered for labor and deliveries.
Anesthesia History
I want to get started by talking about anesthesia history. When people think about historical anesthesia, it is easy to conjure up images of the medicine man pouring a cocktail of some sort that would have mind-altering effects or even someone taking a shot of whiskey or some ancient form of alcohol and told to bite hard on a stick as the doctor worked on a wounded limb. Well, those theatricals are not too far from historical truth.
Early anesthesia is traced back to the true ancient times. The Babylonians, Greeks, Chinese, and Incas are ancient civilizations and all practiced anesthesia. The Egyptians used the combination of opium poppy and Hyoscyamus. A similar combination of morphine and scopolamine is still used for pre-medication today. Incan surgeons were known for chewing cocoa leaves and spitting saliva, presumably containing cocaine, which by the way, is a local anesthetic into the surgical site or wound. Around 1250, Theodoric Borgognoni, an Italian physician and Bishop, soaked sponges with opium and mandragora for surgical pain relief. In 1540 Valerius Cordus synthesized Sweet Oil of Vitriol in Germany by distilling ethanol and sulfuric acid, discovering that this oil had anesthetic properties.
It is important to note that most early anesthetics were inhalational, followed by local, regional, and finally intravenous anesthesia. Intravenous anesthesia was late to the scene because the hypodermic syringe and needle were not invented until 1855. It was destiny that the first eight anesthetics were inhalational.
As mentioned earlier, the Sweet Oil of Vitriol was later called Ether. At the time of its early discovery, its true potential was largely untapped. Ether was used by the medical community mainly for frivolous purposes. Traveling lecturers would dispense ether to a volunteer from the audience to test its mind-altering effects. These early frivolous uses are known in history as ether frolics. In 1842, William E. Clark and Crawford Long used ether independently on patients for various surgeries. However, they did not publicize this discovery. Such a shame is that most sources do not credit these two surgeons.
It was not until four years later that William TG Morton conducted the first publicized demonstration of general anesthesia using ether. The success of that exhibition led the operating surgeon to exclaim to an audience of skeptics, "Gentlemen, this is no humbug." Despite its early use, due to underutilization and lack of understanding, it only truly evolved in the mid 19th century and became firmly established less than six decades ago. The development of surgical anesthesia is considered one of the most important discoveries in human history.
Does anyone know who discovered oxygen? Historically, English chemist Joseph Priestley is credited with independently discovering oxygen in 1774 by the thermal decomposition of mercuric oxide in isolation. However, it is a trick question because Swedish chemist Carl Scheele likewise made the same discovery in 1772, two years prior, but Priestly publicized his findings first. Maybe this would be the equivalent of today, who posted on social media first. Thus a brief history of anesthesia is explained.
You might be asking, What's anesthesia got to do with it? Truthfully, a lot. My realization that there needed to be some content that bridges the gap between anesthesia and respiratory therapy did not come from my three years working as an ICU nurse alongside respiratory therapists (RT). It came from recently working with the courageous respiratory therapists who volunteered to work in our hospitals' COVID unit during this historical pandemic. As a young ICU nurse, I was too inexperienced to understand the actual value that respiratory therapists bought to the table. Sure, I knew the profession was knowledgeable and an essential part of the critical care team, but working side by side over the past year, I have grown a new appreciation for the ingenuity of the RT, the adaptability, the vast knowledge base concerning ventilator management of incredibly sick patients. I cannot tell you how many times our RTs would find ways to make equipment work or sort of “McGyver” things to function in times of shortages. When the system became stressed, the RT was not. The world of critical care is lucky to have such a valuable profession at its side.
Now begins our journey into anesthesia. We will start by talking about airway equipment, walk down the path of airway management techniques, discuss how pharmacology works and how it is used to help optimize the patient for intubation and ventilation. I will try to paint a picture of certain pitfalls you might encounter and discuss ways to navigate your way out of them, with preparation being the most crucial tool in your toolbox.
Airway Equipment
What do we need in our toolbox? In other words, if you are "it" at your hospital and you are called to an airway emergency for intubation, what are your essentials? I understand every person's different and how they practice or their approach. Some hospitals or institutions have certain medical personnel who are designated to intubate patients. However, for this presentation, I will assume you are intubating either alone or with me. Let's talk about the essentials we see in Figure 1 on airway equipment.
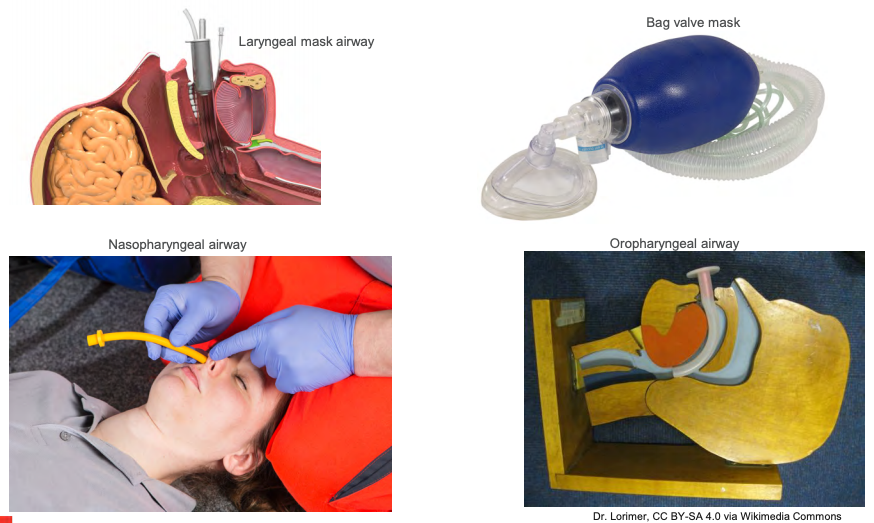
Figure 1. Airway Equipment.
A bag valve mask or Ambu bag is first. This fundamental piece of equipment. The ability to ventilate a patient should never be undervalued because an experienced provider can properly oxygenate and ventilate a patient until a secure airway device is placed into the trachea. Depending on where you are called, I always hope a suction setup is available, but sometimes this can be a luxury item if the patient needs assistance in an area where suction is not available, like a hallway, if they collapse outside of their room, or even in route to a procedure. For this scenario, let's assume we are needed in a patient's room, and we have the luxury of a suction apparatus. If we have difficulty bagging, always have an oral and or nasal airway available. If there is any doubt that we are not achieving adequate oxygenation and ventilation, including oxygen saturation is 70%, minimal condensation in the mask, chest rise is less than optimal, and suspect obstruction, always place an oral or nasal airway.
Genioglossus Muscle
Most times, in the anesthetized or unconscious patient, there is a degree of loss of airway muscle tone or weakness of the genioglossus muscle (as shown in Figure 2).
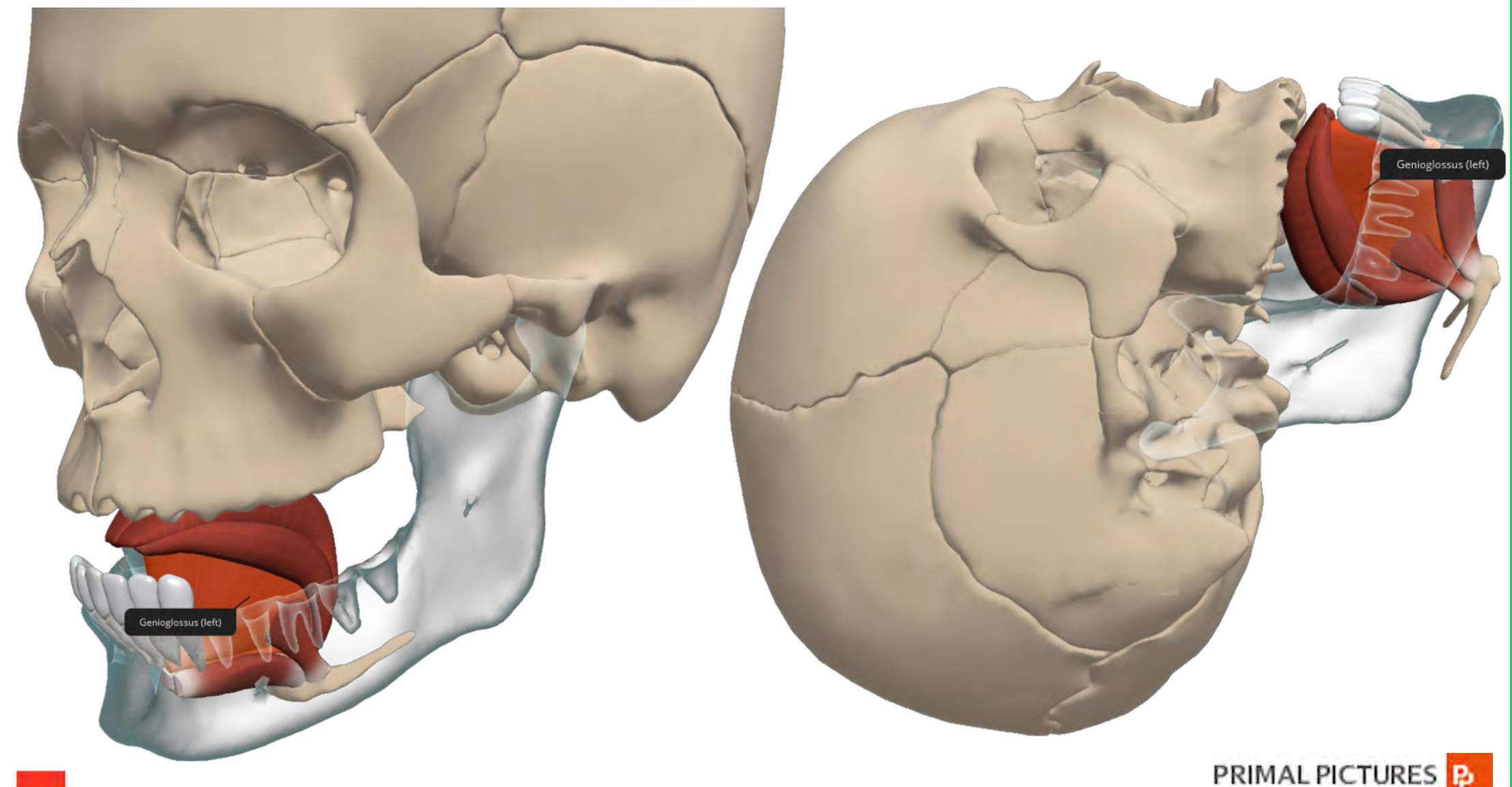
Figure 2. Illustration of Genioglossus muscle.
This weakness allows for airway collapse, and you can see the picture in Figure 2. More specifically, the tongue and epiglottitis fall back against the posterior wall of the pharynx, rendering the patient unable to oxygenate or ventilate. The preferred method to offset this obstruction is repositioning the head or adding a jaw thrust. However, the placement of an oral or nasal airway and repositioning can help maximize airway patency and make life easier while other airway equipment is being prepared for actual intubation.
It is crucial to remember that a patient who has intact laryngeal reflexes may cough or even develop laryngospasm during oral or nasal airway insertion. If the decision to intubate has already been made, medication to help blunt this reflex is recommended. Because of the risk of epistaxis, a nasal airway should not be used in anticoagulated patients or patients with baseline skull fractures. The nasal airway should be lubricated and advanced long the floor of the nasal passage. Frequently we will see someone aim towards the apex of the nasal passage. This approach can lead to traumatizing the turbinates or roof of the nose and causing a bloody airway that is going to make things more difficult.
It is critical to place an airway device in patients smoothly. The placement of an oral airway can be facilitated by using a tongue depressor and selecting the correct size. You can "guesstimate" the size, but there is some evidence that reveals an easy way to determine the appropriate size for patients, and it does not require using a ruler measuring tape.
Oropharyngeal Airway Size in Adults
Research by Kim et al. (2016) supports a quick measure for the maxillary incisors (as shown in Figure 3). The angle of the mandible can help in your choice of size.
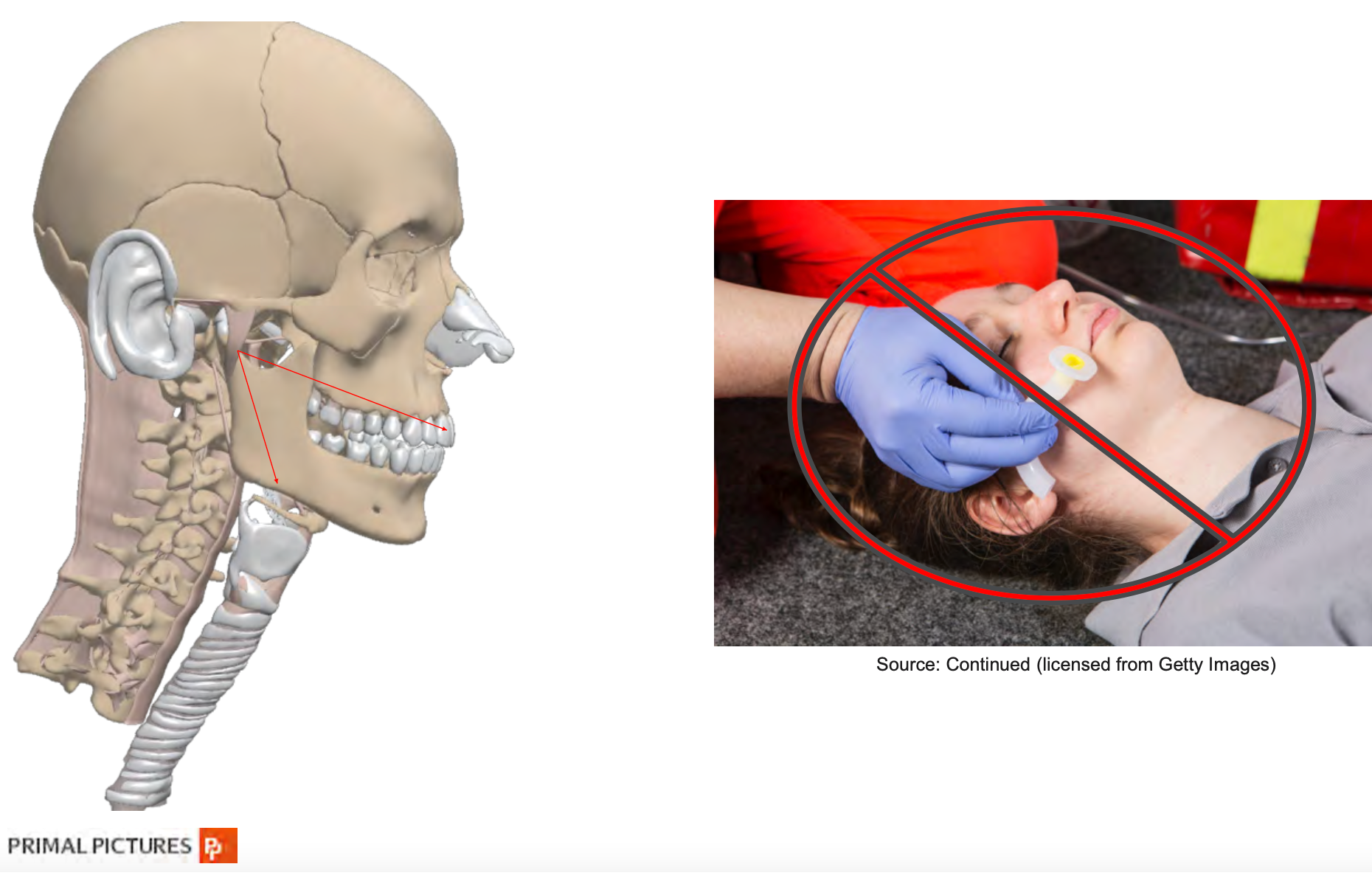
Figure 3. Oropharyngeal Airway Size in Adults.
If you are too short, you may not be behind the tongue, and if you choose a size that is too large, you may further worsen obstruction as it will bunch the tongue back into the oral pharynx. The nasal airway length is determined by measuring the distance from the nares to the meatus of the ear. It should be approximately 2 to 4 centimeters longer than oral airways because you have more ground to cover when approaching the nasal route.
Bag-Mask Ventilation (BVM)
In our scenario, we have taken our quick measurement and placed an oral airway—let's get to ventilating more effectively. The technique is critical. Often this essential step is lost in the chaos of the situation. The patient is coding, and chest compressions move the head and body; a room with multiple people can be overwhelming, including sensory overload, and focus can be easily lost. It is important to remember that your focus should remain on the task at hand, airway support. We always need a tight mask fit. The mask is held against the face by exerting downward pressure on the mask body by the left thumb and index finger (as shown in Figure 4).
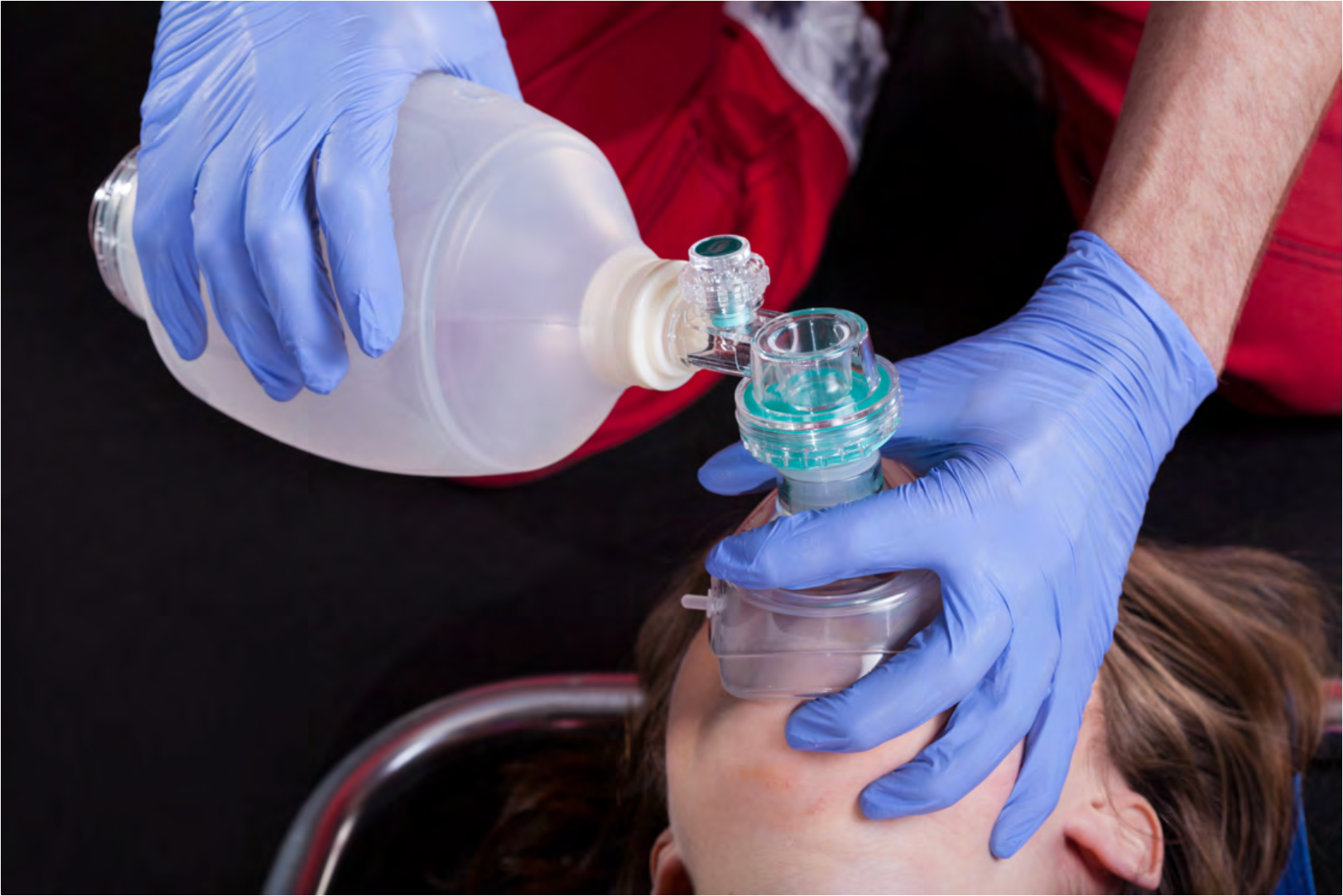
Figure 4. Bag-mask ventilation (BVM).
The middle finger and ring fingers should be grasping the mandible to help extend the atlantooccipital joint, a key point. Keep your fingers on the bone and avoid the soft tissue. This subtle technique maneuver can help to avoid further airway obstruction. Let's not forget the pinky. Place it under the angle of the jaw and thrust it anteriorly towards the ceiling. Never will your pinky feel more important. In certain situations, a two-hand mask technique is warranted for the morbidly obese patient, the patient with a beard, or with a large head.
Airway Management
Another device aiding ventilation is a laryngeal mask airway (LMA). The LMA is not an airway security device. These are not popular devices in a code situation or any situation that requires proper airway security. Think of it as more of an invasive way to mask-ventilate. It also carries with it the increased risk of gastrointestinal aspiration. Regardless, it is certainly something I keep in my toolbox when responding to airway emergencies or in preparation for a possible difficult airway. Each operating room has an abundant supply of various sizes in the hospital I work in because you never know when you might need it. Even some surgeries requiring general anesthesia can be done with an LMA, but of course, these are on patients that have been NPO (nothing by mouth) for at least eight hours.
If you ever see an LMA used in an airway emergency setting, things are likely not going as planned. Moreover, the placement of the LMA indicates a pathway for ventilation for a patient, which is not easy to intubate. It serves as a temporizing measure in patients with difficult airways. The advantages of the LMA are:
- Being easy to insert and has a relatively high success rate of 95- 99%
- Being used as a conduit for an intubating stylet - bougie, flexible bronch, or an endotracheal tube
- Available in both infant and adult sizes
In our scenario, we are proving we are effectively ventilating and oxygenating our patient. Our oxygen saturation is acceptable. We see chest rise. We have end-tidal CO2 monitoring connected and see an acceptable range of CO2 exchange. The patient remains obtunded. There is some degree of hemodynamic instability. We can occasionally see an effort to breathe, but the decision is made to intubate.
Endotracheal tube
What else do we have in our toolbox? The pinnacle of airway security is the endotracheal tube. As a CRNA, this is the airway device I place most often. A fundamental piece of equipment that should not be taken for granted. Known as the "breathing tube," the windpipe that goes into the windpipe. In my practice, various tube sizes are available in the operating room or when responding to emergencies.
Endotracheal tubes are not a "one size fits all tool." The standard endotracheal tube has a beveled tip, which you can see here, to aid in visualization and insertion through the vocal cords. Also, you notice that the side hole called Murphy's eye, located at the distal end of the tube. The Murphy's eye helps decrease the risk of occlusion if the distal tube abuts the carina or trachea.
Some formulas help determine internal diameter (ID) tracheal tube size, but 7 to 7.5 ID is generally acceptable for most adult females. Adult males usually require 7.5 to 8 ID, and even up to 9 ID. Usually, I see around 7.5 to 8 ID. If you examine the endotracheal tube, you will most likely notice a radiopaque line, which is when you want to confirm tube position on a chest X-ray—another endotracheal tube fun fact. You will also notice it as a natural curve to it. The Magill curve was a happy accident. When Sir Ivan Magill first used the single lumen rubber tubes for nasal tracheal intubation in the 1920s, he cut them from a coil of tubing; hence they were curved. He found the curve helpful. This natural curve allows for the omission of an intubating stylet in most patients.
In a highly controlled environment during training, most anesthesiologists and CRNAs do not routinely use a stylet for intubation in the operating room (OR) setting. Some training facilities prefer individuals training for intubations not to use a stylet as it creates a more rigid distal end, and the novice tends to "saw" or move back and forth the endotracheal tube as they are trying to get it into the trachea, causing airway trauma. I highly recommend using a stylet for any airway situation outside of the OR setting, precisely any airway emergency or code situation, because it is in the endotracheal tube if you need it, locked and ready to go. These patients are already compromised and usually have little oxygen reserve. The loss of valuable seconds because the stylet is not within the tube means further patient compromise. I am not willing to bend that rule. No pun intended.
Laryngoscopes
Back to our scenario, we decide to intubate our patient. We have a male patient of average size. We have our endotracheal tube ready with a stylet in place and the cuff intact, checked for integrity. What else do we need? We need the laryngoscope (as shown in Figure 6).
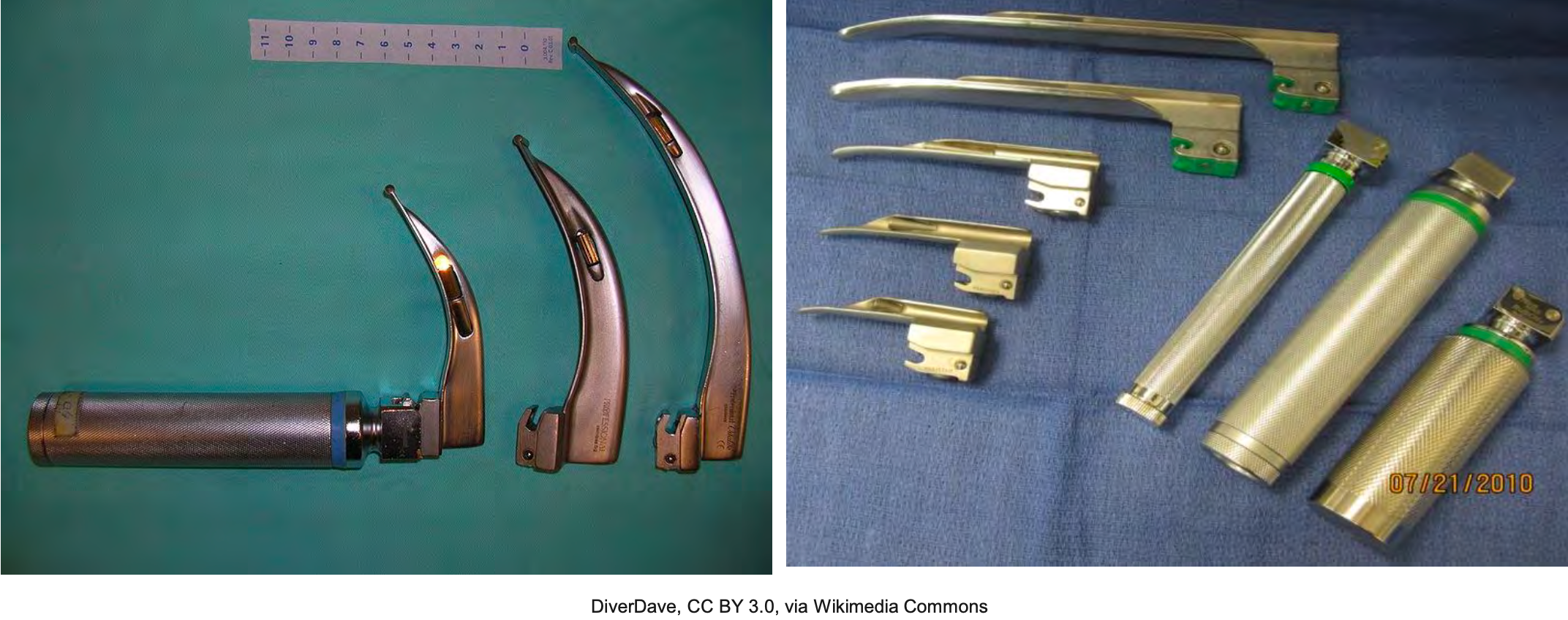
Figure 6. Types of Laryngoscopes: Mac and Miller blades.
As we prepared, we have the most commonly used one. Herein lies an age-old question, Miller or Mac? This debate always makes me laugh a little bit. If you have ever listened to the argument by two seasoned laryngoscopists, each person will sound very passionate about why their blade is superior to the other. At the end of this debate, you will be convinced that both are superior blades. I will not get into this one too much, but I have discovered that the best blade is the one you are most comfortable with. Whoever is intubating, the RT, anesthesiologist, CRNA, pulmonologist, EMT, paramedic, should learn how to use and be proficient with both and then determine which they like best.
It is very subjective. The main difference, the Miller, is straight, and the Mac is curved. The Miller is placed underneath the epiglottitis. In contrast, the Mac is placed in the vallecular space to visualize the airway. In the last decade, Mac's direct laryngoscopy methods, e.g., videoscopes, have become popular, and some circles argue it will become the intubation standard in the future.
Glidescope
At the institution I work in, we have the GlideScope (as shown in Figure 7).
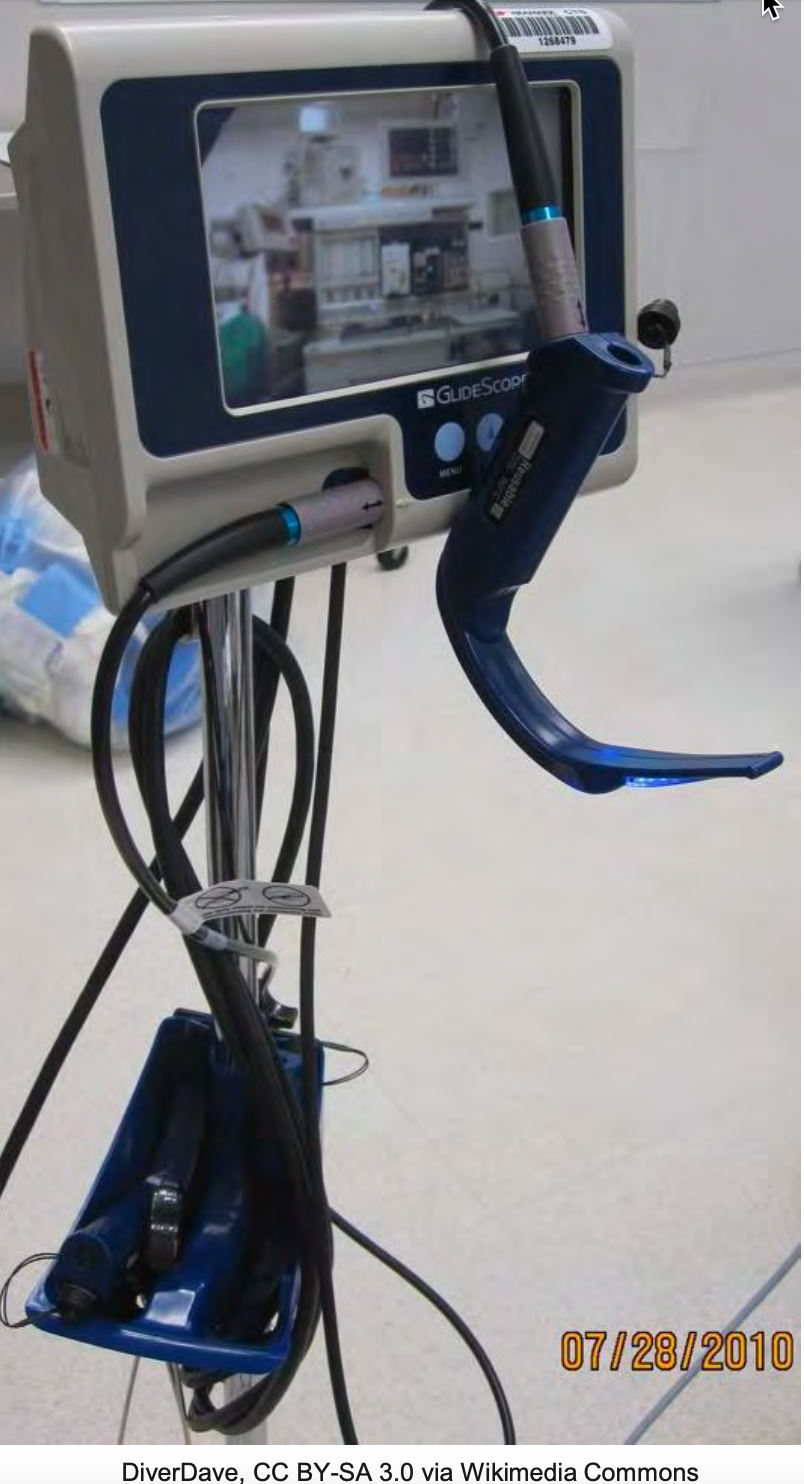
Figure 7. Example of a Glidescope.
The GlideScope is one variation of a group of videoscopes available, but this is the one that I am most familiar with. A videoscope is an excellent adjunct tool to have when there is little time to assess the patient's airway, such as a code situation or emergency. Evidence suggests that using the GlideScope yields a success rate of 90% within 120 seconds, within the first attempt, even for untrained personnel. In this particular study (Nouruzi-Sedeh, Schumann, & Groeben, 2009), these personnel only had mannequin training before human attempts. The evidence for the use of the GlideScope is compelling and certainly supports its use. In my practice, we always have a GlideScope available or within reasonable reach in the OR area, and we always take one with us to emergency airway calls. It is one of those things where it is better to have it and not need it than to need it and not have it.
Something I have found to be very profound as well, as mentioned, the anesthesia department where I work is responsible for airway calls throughout the hospital, including the emergency department. Before getting the GlideScope, the emergency department would call us at least once or twice a week for help with intubations. When they finally purchased the GlideScope, these calls dropped significantly. We might get a call from these guys, maybe once every two to three months. We know if they are calling us, the airway is genuinely difficult.
Pharmacology: Induction Agents
Let's say we have done all the things. Our patient is safely intubated, and for the RTs, security of the airway device and ventilator management becomes paramount. Thank goodness you have all the tips and tricks in airway security devices. In the operating room setting, CRNAs do a great job securing the endotracheal tube, but let's be honest, RTs do the best job. Our patients are under general anesthesia in the operating room, and patient movement is not an issue until it is time to extubate. There is less time or somewhat less of a chance of inadvertent extubation. In the ICU, it is an entirely different ball game. There are spontaneous breathing trials, prolonged weaning from the ventilator, awake intubated patients who may be uncomfortable, delirious, or agitated. The airway security device is critical and leads us to the next topic.
Our patient is safely intubated, an airway security device is in place. What is the best way to keep our patient comfortable on the ventilator? The answer is quite simple- pharmacological agents, anesthetic agents. Coming from an anesthesia background, in the OR, we have the benefit of inhalational agents. No, not ether but more modern and safer agents. I will talk a little bit about them later, but since you will not be seeing them, we will talk about the everyday things you will see outside of a surgical area. I recommend going over pharmacology and figuring out what agents are best for patient comfort while on the vent.
Back to our patient that we just intubated, first ask, what is still lingering from the intubation? Yes, we gave some medication for the intubation, but I wanted to keep the discussion of pharmacology in one area and keep the dialogue less convoluted. What is still lingering from the intubation? It will likely be a hypnotic sedative agent, specifically Etomidate, Propofol, or Ketamine combined with a paralytic agent. In most cases, the paralytic will either be Succinylcholine or Rocuronium.
Why are we using these agents? Our patient was semi-conscious or obtunded at worst. Will the patient remember anything? Well, we are not sure. Recall memory is hard to assess at that point, and we have to ensure safety and comfort for the patient before intubation. In this scenario, we used medication for intubation. Keep in mind that there will be times where medication is not necessary. Such as in the patient who is actively coding, but each situation is different. Again, in our case, we chose to medicate. Medication will aid in the optimization of the airway for intubation, and the choice of medication has to be tailored specifically to each patient. It should not be a cookbook or recipe-type thing. Ideally, you will need to ensure three things before intubation:
- Amnesia
- Analgesia
- Muscle relaxation
Amnesia will come by rendering and guaranteeing that your patient is unconscious with Etomidate, Propofol, or Ketamine agents. These are your brain scramblers, and we will talk about them in-depth shortly.
What is GABA?
Before we delve into it, I would like to mention one crucial neurotransmitter and receptor. You will hear it mentioned from time to time, and I want to ensure you have some detailed knowledge of it so what I am saying makes a little more sense. The important neurotransmitter and receptor is called the Gamma-aminobutyric acid or GABA (as shown in Figure 8).
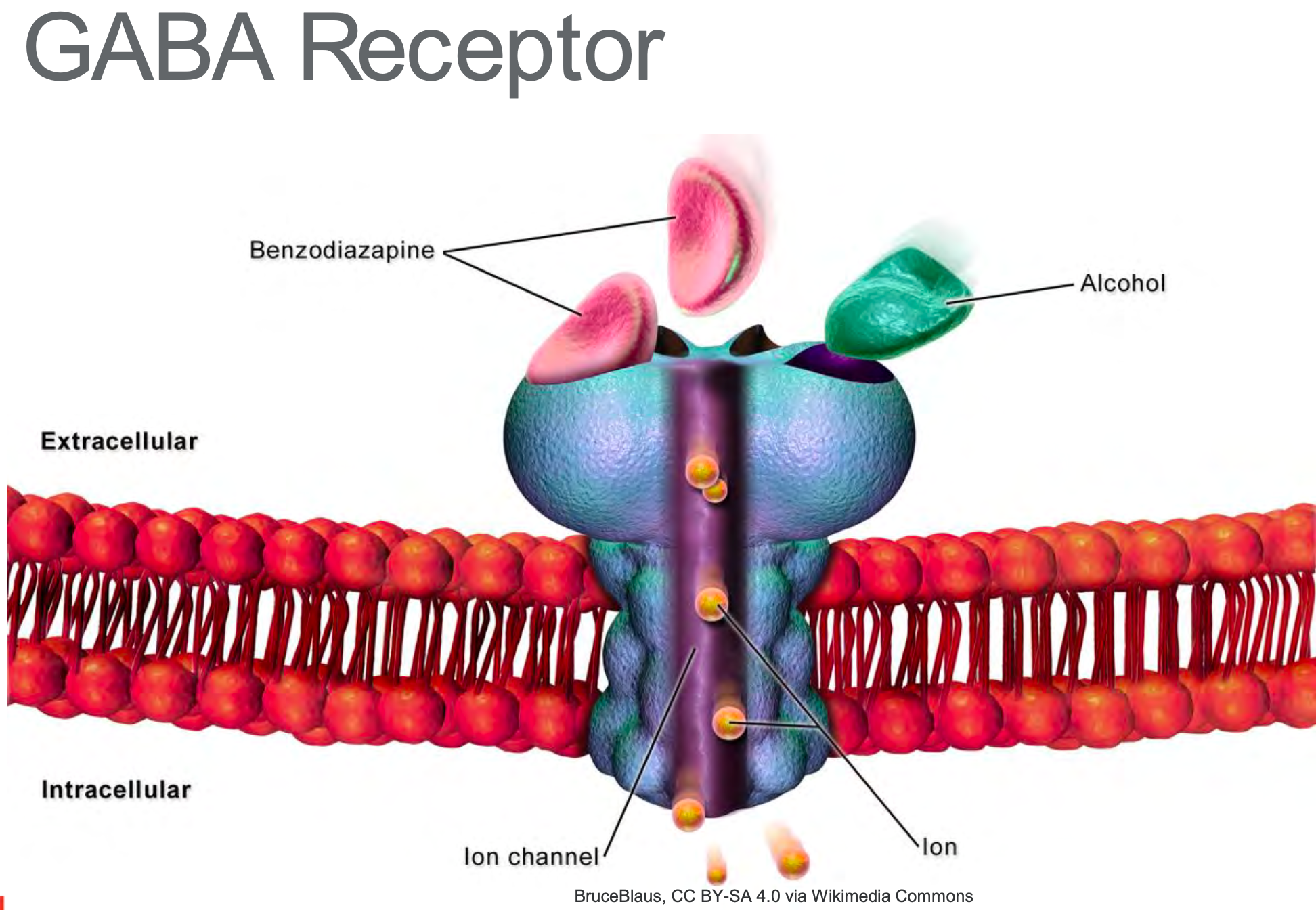
Figure 8. GABA Receptor
GABA is the primary inhibitory neurotransmitter in the brain and is a major inhibitory neurotransmitter in the spinal cord. Many anesthetics enhance GABA inhibition of the central nervous system. GABA receptor agonists appear to enhance anesthesia, whereas Gabba antagonists reverse some anesthetic effects. When you hear GABA, understand things are slowing down, and your neurons are less likely to fire on the action potential. For example, ever hear someone talk slower after a few glasses of wine? You can thank GABA for that.
Etomidate
Etomidate depresses the reticular activating system and mimics the inhibitory effects of GABA. It also may have disinhibitory effects on parts of the nervous system that control extrapyramidal motor activity. This inhibition offers a potential explanation for the 30-60% incidents of myoclonus with its use. What you might see is mild to profound muscle jerks or tightening shortly after Etomidate administration. The recommended dose for intubation is 0.2 to 0.5 milligrams per kilogram IV.
The benefit of Etomidate is it has minimal effects on the cardiovascular system. A mild reduction in peripheral vascular resistance is sometimes responsible for a slight drop in blood pressure, but I have rarely seen that.
Cardiac output and myocardial contractility are usually unchanged, and it does not release histamine. In terms of respiratory effects, even induction doses do not usually result in apnea. Some intensivists are concerned because it can cause adrenal suppression, but it is transient with a single dose. This medication is ideal for your cardiac patients who have heart failure or hemodynamically unstable, or both. Although you may not see apnea with a second dose, it can sometimes cause respiratory depression.
Propofol
Our second agent, Propofol, is probably the most widely used induction agent for intubation in the OR setting. Outside of the OR critical care areas, these drugs have to be used judiciously. It will induce a state of general anesthesia, and in my world, it is sometimes called Milk of Amnesia because of its milky white appearance. It works by facilitation of inhibitory neurotransmission mediated by GABA.
Usual induction doses range from one to two and a half milligrams per kilogram IV. It has a rapid onset and relatively rapid recovery time. The significant cardiovascular effect of Propofol is a decrease in arterial blood pressure due to a drop in systemic vascular resistance, cardiac contractility, and preload. May see hypotension after its use, after induction for intubation. Factors exacerbating the hypotension include large doses, rapid injection, and administration to the elderly. That is why the dose range mentioned previously is so broad. The older you are, the less you get, the sicker your heart is, the less you get, especially if you have heart failure and compromised heart function.
Propofol's respiratory effects are profound, and apnea occurs after an induction dose. Even when used for conscious sedation and subanesthetic doses, a Propofol infusion inhibits hypoxic ventilatory drive and depresses the typical response to hypercarbia. Although Propofol does not induce a histamine release, it is not contraindicated in patients with asthma as the histamine release is pretty insignificant. Of course, these side effects make Propofol sound dangerous, but with proper dosing, its safety profile is excellent, and it is ideal for most patients, especially when you are trying to achieve an apneic state before intubation.
Ketamine
Finally, we have Ketamine. Ketamine has multiple effects throughout the central nervous system, including blocking polysynaptic reflexes in the spinal cord and inhibiting excitatory neurotransmitter effects in selected areas of the brain. Ketamine functionally dissociates the thalamus from the limbic cortex, which is involved with the awareness of sensation. Although some brain neurons are inhibited, others are tonically excited.
What might you see clinically? Most patients appear conscious, have open eyes, can swallow, and have muscle tightness but cannot process or respond to sensory input. After administration of Ketamine for intubation, a glazed look on the patient appears as it takes effect. They may not close their eyes like they do with the Etomidate or Propofol. They may continue to stare out into space until the paralytic hits their system.
A quick anesthesia fun fact, Ketamine is a structural analog of phencyclidine. Does anyone know what phencyclidine is? Well, this is also known as angel dust. It is 1/10 as potent yet retains many of phencyclidine’s psychotic-mimetic effects. Even sub-therapeutic doses of Ketamine can cause hallucinations. Cardiovascular effects are quite the opposite of what we see with typical induction agents. We usually expect some drop or at least minimal change in hemodynamics, as discussed earlier with Propofol and Etomidate, but Ketamine is unique and sometimes casually referred to as Special K in the anesthesia world.
It increases arterial blood pressure, heart rate, and cardiac output. These indirect cardiovascular effects are due to central stimulation of the sympathetic nervous system and inhibition of the reuptake of norepinephrine. It is going to make the patient's heart work a little more. For these reasons, we try to avoid it in patients with coronary artery disease, uncontrolled hypertension, congestive heart failure, and unknown arterial aneurysms. Indirect stimulatory effects of Ketamine can prove beneficial to patients with acute hypovolemic shock.
From a respiratory standpoint, Ketamine's effects are well-established. The inhibitory drive is minimally affected by the usual induction doses of Ketamine. That induction dose is 1-2 milligrams per kilogram. A quick side note, doses needed for conscious sedation can be a great adjunct to the anesthetic because it minimally affects ventilation. Another thing that makes Ketamine unique is its known bronchodilator effects, making it the ideal induction agent for asthmatic patients. We might give it to patients who exhibit signs of bronchospasm post-intubation in the OR, and I have seen it work nicely. Another advantage is that Ketamine has analgesic properties as well. One of the drawbacks of this particular anesthetic is its ability to increase patient salivation. You might see increased secretions if this drug is used anytime during a patient's care.
Sedation Strategies
We have discussed some common induction agents, simply put, a stabilizer, a potential downer, and a stimulator, Etomidate, Propofol, and Ketamine, respectively, each with their unique characteristics can help provide optimal intubating conditions for the patient. Of course, we will add a muscle relaxant for our intubation. We will talk about that more in-depth later.
Let's get back to that guy. Remember the one you intubated smoothly. His heart was healthy, but because he had hemodynamic instability early on, you chose Etomidate, which worked well, but the Etomidate will not last forever. It is only a matter of time before he starts coughing and "bucking" the vent. How will we make him "like being on the vent" and keep him comfortable for his condition to be worked up and resolved? Again, more pharmacology.
Benzodiazepines
Let's talk amnesia. Breathing through a straw can be very stressful, an event most individuals do not want to remember. That leads us to a commonly used drug in the ICU, the group known as Benzodiazepines that interact with specific receptors in a central nervous system, particularly in the cerebral cortex. Benzodiazepine receptor binding enhances the inhibitory effects of various neurotransmitters. For example, the Benzodiazepine receptor binding facilitates GABA receptor binding, increasing chloride ion's memory conductance.
What does this all mean? It means that, again, things slow down, and it is harder for neurons to fire. Common Benzodiazepines include Versed (Midazolam), Ativan (Lorazepam), and Valium (Diazepam), and you will commonly see Versed, or Ativan drips in the ICU setting post-intubation. Effects on the cardiovascular system are minimal. Although it is not uncommon to see a slight decrease in blood pressure, a patient is more relaxed and less anxious, making sense. Effects on heart rate are variable but will often see the heart rate unchanged or slow down a little. Respiratory effects from Benzodiazepines, we know they depress the ventilatory response to CO2.
Your patient gets a big bolus of Versed, guess what? They may not breathe faster if their PCO2 rises. It could be good or bad, depending on what you are trying to achieve in the ICU. IV Benzodiazepines, in particular, are known respiratory depressants, and patients on Versed can even become apneic. This is great if you are trying to breathe for someone with sick lungs. Remember, we want the patient to “like” our vent settings because they were most likely intubated for a significant reason after all. We want to maximize comfort and minimize ventilator-associated lung damage.
Opioids
What other drugs will you see in combination with Benzodiazepines? Well, I did bring up comfort. How are we going to reduce the discomfort and/or pain of ventilatory support? Well, we need pain medicine. That brings us to the class of drugs known as Opioids. Fentanyl, Dilaudid, or Morphine infusions are pretty standard in the ICU. in particular, at my hospital, Fentanyl is usually the go-to. It is important to keep in mind that all Opioids have some sedation effects. They are most effective at producing analgesia. There are four major types of opioid receptors, Mu, Kappa, Delta, and Sigma. The pharmacodynamic properties of specific Opioids depend on which receptor is bound, the binding affinity, and which receptors are activated.
In general, Opioids do not seriously impair cardiovascular function. High doses of morphine and Fentanyl are associated with vagus-mediated bradycardia. That is why you might see the patient get bradycardic after a Fentanyl bolus, and it is producing its analgesic effects. It helps to attenuate the adrenergic response to stimulation. In our case, that stimulation is the vent in the endotracheal tube. Picture your patient in ICU on Versed and Fentanyl drips, the combination working synergistically, and with the addition of the Fentanyl, you see some bradycardia, venodilation, and decreased sympathetic reflexes. It is not uncommon to see blood pressure fall a little bit in patients supported on low-dose vasopressors. At least at my facility, there has been a trend of seeing fewer morphine infusions being used. I have not seen it used in years.
Fentanyl and Dilaudid have a reputation for being cleaner drugs in terms of clearance and side effects. Morphine also evokes histamine release, and it can lead to profound drops and systemic vascular resistance and arterial pressure, decreasing its popularity because its ability to depress ventilation is excellent for patients on the ventilator, particularly in patients who need full ventilatory support for sick lungs. The drive to breathe despite increased PCO2 is blunted with Opioids—also, the hypoxic drive decreases. The respiratory centers in the brainstem mediate these effects. It is also important to note that Opioids can induce chest wall rigidity severe enough to prevent adequate ventilation; this usually occurs with substantial doses not commonly given in the ICU. I have never seen it, but if we ever did see it, the treatment is a paralytic agent, which, again, we will talk about shortly. Finally, I would like to mention that Opioids can also blunt the bronchoconstrictive response to airway stimulation during intubation, making Opioids ideal drugs for pre-intubation or even "bolusing" for bronchoscopy in the ICU. You will notice lower peak pressures as bronchoconstriction resolves.
Dexmedetomidine (Precedex)
Another anesthetic agent you might see for sedation that is not used for standard and intubation but will undoubtedly be used to help with mechanically ventilated patients is Dexmedetomidine or Precedex. Precedex is a centrally acting highly selective Alpha2 adrenergic agonists properties that proved very useful in the intubated patient.
First, it has potent sedative and analgesic expiring properties. In therapeutic doses, it does not cause respiratory depression, making it extremely attractive for infusion sedation. However, it causes reduced sympathetic outflow. Blood pressure and heart rate drops can be seen. Because of its properties, it is a valuable agent in the ICU. Patients can even be weaned from other sedatives and mechanical ventilation faster with it on and be extubated without discontinuing this infusion. I mentioned the Opioid. We can reduce Opioid use in a patient on Precedex, subsequently avoiding unneeded respiratory depression when we need it from the ventilator.
Back to our patient, we now have a few drugs in our arsenal working for him, Versed, Fentanyl, maybe Precedex if he needs another. You can try a combination of all the medications we discussed, in particular Propofol and or Ketamine. Sometimes Propofol can be used alone if you anticipate a short intubation period due to relatively rapid recovery. However, our patient is sick. His lung compliance is worsening, showing signs of acute respiratory distress syndrome (ARDS). Our vent settings are unnatural with lung protective strategies in place.
Neuromuscular Junction
Despite all we are doing with pharmacology, he is dyssynchronous on the event, he is “desatting” despite sedation boluses your ABG is looking worse, you notice occasional bucking on the vent, and with each cough, there is significant desaturation. He is not happy with what we require on the vent for him. Along with fewer collaborations, the team decides he is receiving enough sedation and pain control. At a certain point, you and your ICU team need to consider neuromuscular blocking agents or paralytics—first, a little background on how these agents work and where they work with regular neuromuscular transmission. The neuromuscular junction is a region between a motor neuron and a muscle cell (as shown in Figure 9).
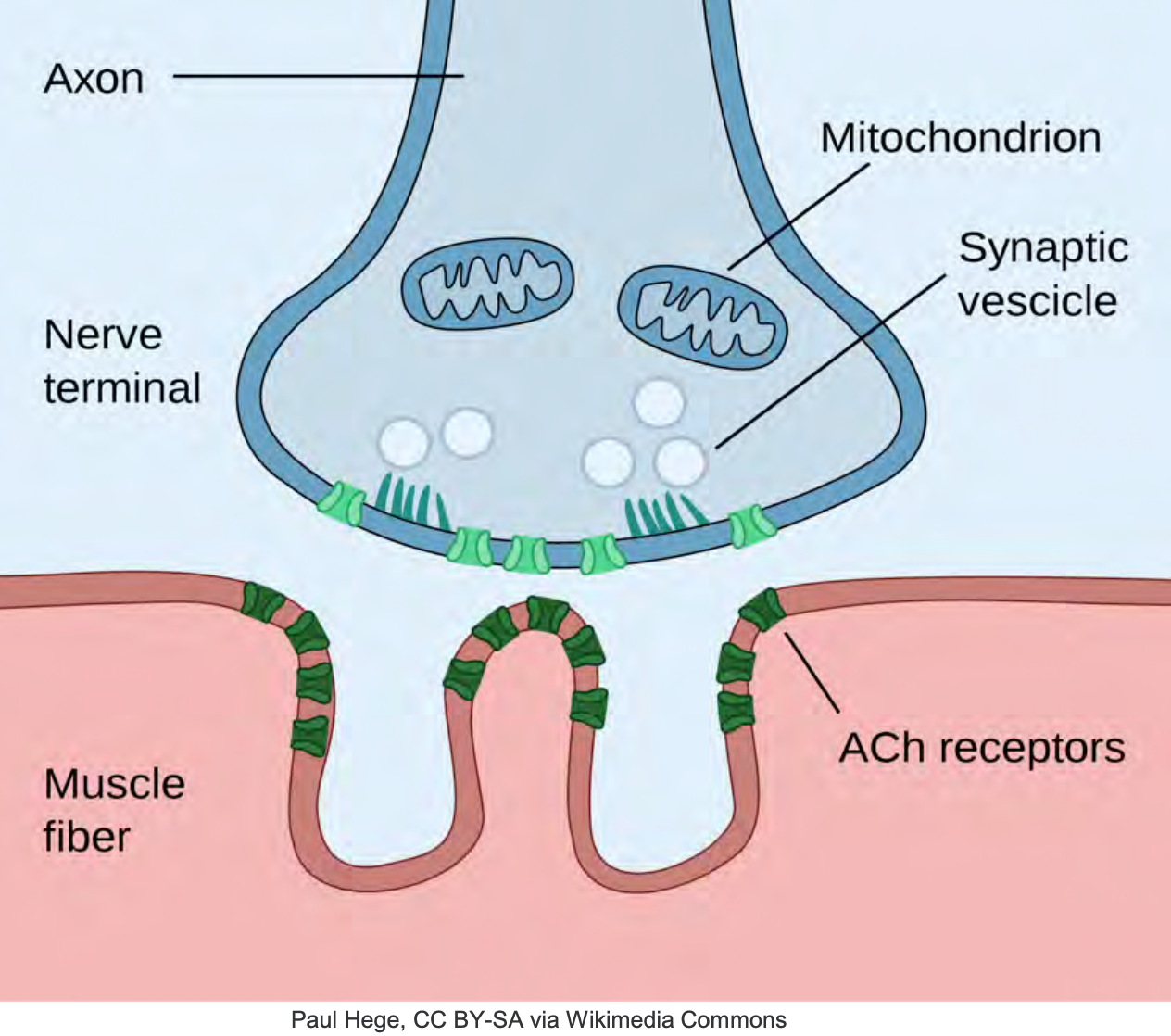
Figure 9. Illustration of the Neuromuscular Junction.
Cell membranes of the neurons and muscle fiber are separated by a narrow gap called the synaptic cleft, which you will see right here. The nerve action potential and various ion release, acetylcholine molecules, a neuro-transmitter, diffuse across the synaptic cleft to bind specific areas on the muscle membrane, the motor endplate. Right here, you'll see these acetylcholine receptors. Once an inflate potential is generated, the muscle here contracts, the breakdown of acetylcholine by an enzyme, acetylcholinesterase, eventually causes the muscle to relax. So our neuromuscular blocking agents are divided into two classes, the polarizing and nondepolarizing. The difference lies in the mechanism of action.
Succinylcholine
Let's go back in time to the guy we intubated. Once we decided to intubate, someone in the crowded room shouted, Can someone grab the succs? Succinylcholine is our only depolarizing muscle relaxant, similar to a seat of acetylcholine which causes the muscle to contract or depolarize. Succinylcholine will occupy the receptor constantly stimulated, supercharge the postsynaptic membrane and cause action potentials to fire over and over again. By doing this, they essentially tire out the postsynaptic membrane. This excitement to rephase is going to present as muscle contraction to you. We call these contractions fasciculations and will present as mild to intense depending on your patient's size or muscle mass.
I have had some elderly patients who may show little to no fasciculations and young adult females or males with strong ones. When I say strong, I mean pretty impressive, almost looking like they are trying to wiggle their way off the bed. Once after Succinylcholine administration, I had a physician at the foot of the bed shout, Hey, this guy tried to kick me. The popularity of Succinylcholine is due to its rapid onset of action, about 30 to 60 seconds, and its short duration of action, around 10 minutes, making it ideal for intubation. Despite its many advantages, it has some drawbacks to keep in mind because normal muscle releases enough potassium during Succinylcholine-induced depolarization to raise serum potassium levels by about 0. 5 milliequivalents per liter. It can be life-threatening and patients with hyperkalemia. We see this often in patients with renal disease, burn injury, or massive trauma, among other conditions. If you are ever concerned about a patient's potassium level, you are better off not using Succinylcholine for intubation. Aside from increasing potassium level, it can induce muscle pain, increase intraocular pressure, intracranial pressure, or even induce a life-threatening condition called malignant hyperthermia.
Nondepolarizing Relaxants
Let's say our guy, for whatever reason, had a potassium level of 5.8, and we were alerted he had a history of renal disease. For us, Succinylcholine is ruled out. What do we use? What was our paralytic of choice for our intubation earlier? Well, we played it safe and went with a class of paralytics known as the nondepolarizers. In contrast to our Succinylcholine (depolarizer), there is a wide selection of non-depolarizers, Rocuronium, Vecuronium, Cisatracurium, to name a few. Due to the drug agents' longer duration of action, they are ideal for muscle relaxation during surgery and, in our patient's case, for intubation who has a high potassium level and the need for paralysis to permit de-recruitment from asynchrony on the ventilator.
Why do we even need paralysis for intubation? Paralysis will drastically improve intubating conditions. Your patient will be flaccid, making it easier to ventilate them, their vocal cords will be relaxed, and paralysis guarantees no diaphragmatic movement. The literature on this is convincing. Studies have shown that emergent intubations without the use of paralytic agents lead to an increase in adverse events. More recently, Walls et al. (2011) looked at 8,937 emergency department intubations and found that the likelihood of any associated event was 1.7 times higher with sedation without paralytics comparing to using sedation with paralytics. It is important to note the paralytics do just that. They only paralyze. When administrating a sedative before a paralytic, make sure the onset of your sedative has the same onset of action as your paralytics as there will be no awareness.
That takes us back to our guy. We did that. We gave Etomidate and chose our nondepolarizing agent, Rocuronium, because you will often see it used for rapid sequence induction with the comparable onset time to Succinylcholine, but it will last a bit longer. A rapid sequence induction dose usually lasts about 45 minutes to an hour, depending on the patient's metabolism, but how is it working differently from Succinylcholine? Our nondepolarizers bind acetylcholine receptors and sit there, blocking all activity. To compensate for its longer onset time, we have to increase the standard dose for the rapid sequence induction, at least 1.2 milligrams per kilogram IV, and about 45 to 60 seconds for adequate intubation conditions is the waiting period.
Let's recap. We have done a lot for this patient in a short time. We have opted to intubate. We used our sedative Etomidate then administered Rocuronium, avoiding Succinylcholine due to its elevated potassium level. We knew the Etomidate would wear off within a few minutes, our nursing staff was ready with Versed and Fentanyl drips to maximize comfort, given he was on the ventilator as the Rocuronium that we used for intubation was wearing off. He began to cough, buck the ventilator, and sedation boluses worked until his lungs became challenging to ventilate. As our ventilator settings changed, he was not happy. We opted to maintain sedation and added a paralytic to avoid the recruitment, increased oxygen consumption, and dyssynchrony on the vent. Nice work.
Ventilator Weaning
Days pass and the patient's lungs begin to heal. The paralytic is no longer necessary. We begin to show signs of improvement overall. The set requirements are minimum, so how will we smoothly wean this guy off sedation for his extubation? Well, truthfully, weaning sedation should be tailored to the individual patient. As patient conditions improve, this is something that should be worked on daily. If you were to go on your computer and do a Google search, the yield would be high for various methods. Indeed, an arena of critical care medicine where the opinion-based practice is currently hard to avoid because robust evidence is lacking. I can give you some general guidelines, and my references will point you to a few areas for help, but this entire topic could easily be worthy of another separate presentation.
However, I will say that long-acting medications like benzos should be weaned first, and literature supports that. Remember also that there are medications that you can keep on while working on extubation. In my experience, low dose Opioids and or Precedex are preferable and perfectly acceptable to keep on as long as they do not affect your patient's respiratory status. Another thing to remember is that rapid and abrupt termination of an established sedative regimen should be avoided, primarily when longer-acting sedatives have been used. It could lead to withdrawal symptoms. Frequent signs of withdrawal include agitation, diarrhea, fever, tachypnea lacrimation, and hyperactive delirium. According to one recent study, patients who developed a withdrawal syndrome spent almost double the amount of time receiving mechanical ventilation. It is important to note that some of these symptoms may mimic respiratory distress on the vent that may lead you to think the patient is not ready for extubation. Imperative to use drug strategies to help for a smooth and uneventful wean.
Let's say, for our purposes, our patient is doing fine and as we wean, and his vent settings are minimal. He is doing great with spontaneous breathing trials, his Benzodiazepine has off, and he is following commands. He is on low-dose Precedex, and you alert the nurse when he gets tachypnea mildly. When the nurse gives a small dose of Opioids, in this case, Fentanyl, the patient calms down and continues to pull adequate volumes. Everything points to, hey, this guy is ready to extubate.
What About a Cuff Leak?
Kuriyama & Kamei (2020) concluded laryngeal edema and airway obstruction following extubation is a major cause of extubation failure. Post-extubation stridor, its clinical sign, has a reported incidence of 2–26% and frequently results in reintubation. Reintubation is associated with an increase in morbidity, duration of mechanical ventilation, and ICU stay. The cuff leak test has excellent specificity but low to moderate sensitivity for post-extubation airway obstruction. Your input and the Intensivist both decide the patient is ready, but the nurse says there is no cuff leak (see Figure 10).
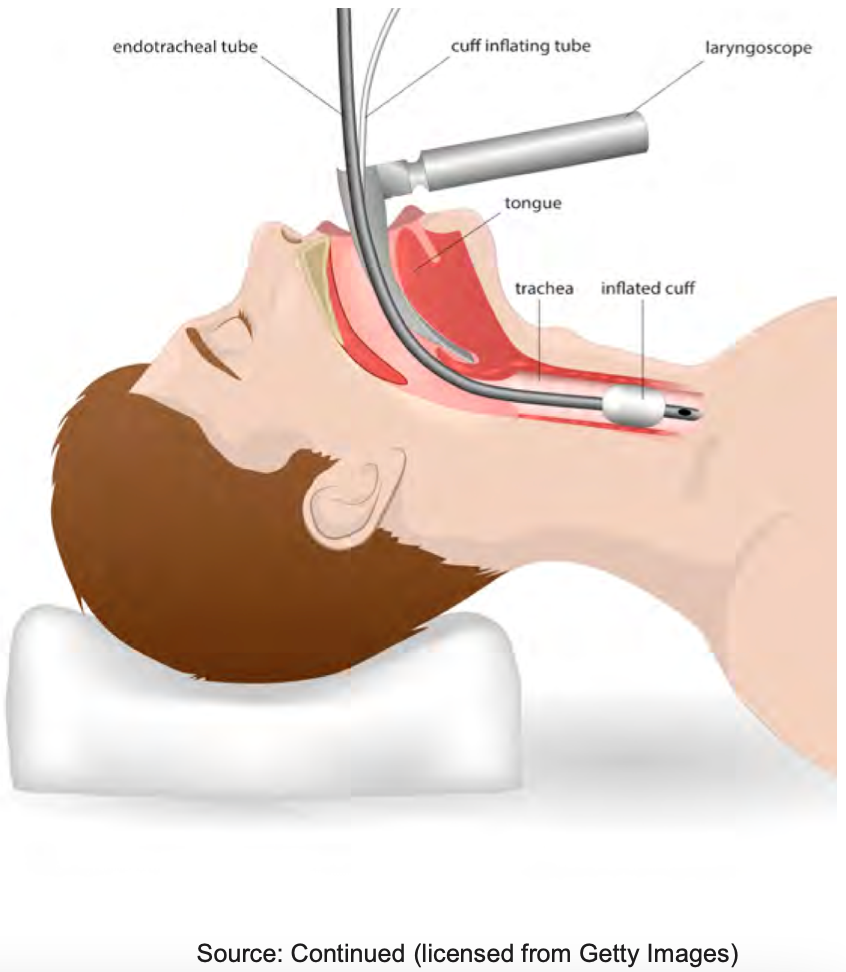
Figure 10. Illustration of an endotracheal tube and inflated cuff.
What are your thoughts? Should you hold off? Well, honestly, opinions vary on this topic. Most evidence suggests that the cuff leak test has excellent specificity but low to moderate sensitivity for post-extubation airway obstruction. It can be a valuable tool in deciding whether or not to extubate a patient, but the low sensitivity suggests that a negative test, meaning the presence of a leak, cannot wholly exclude post-extubation airway obstruction.
Therefore, this test should not be the sole variable in deciding to extubate if all other factors suggest you should. The team and their collaborative efforts should decide if it is safe and factor in other things such as, was the patient a difficult airway upon initial intubation? Does there continue to be airway swelling? Do you think there may be encrusted secretions accounting for no-leak rather than upper airway swelling? My point is here, what your team deems the higher-risk patient, it is probably safer to give steroids and wait, but if they are not, it might be perfectly acceptable to extubate. The global picture and context of your patient's history and condition should be factored in as well
Clinical Pearls of Wisdom
Finally, to wrap it up, some clinical anesthesia pearls. When we, the CRNAs, come from the OR and you are in the ICU ready for us to transfer care, the detailed report should paint a clear picture of what is coming to you. Sometimes we bring intubated patients, extubated ones, wide-awake ones, sleepy ones, and some unpredictable. Ideally, the transfer of care is smooth and uneventful, but sometimes anesthetics can be unpredictable. If the surgery was long, then it is possible that the inhalational agent or anesthetic gas is not washed out of the patient's system. Quite commonly, you will see some mild tachypnea that is directly related to the inhalational agent. But this should resolve within a few minutes, something you to be aware of. So back to the toolbox. I mentioned a few things I keep handy, but there is so much more, keep in mind for this presentation. In our airway scenario, I talked about what we used and the must-haves, but we are never limited to those. Fiber optic scopes, bougies, colorimetric detectors are some other things I know are immediately available to me. You need to become familiar with what you have available at your facility. The last thing, in any clinical situation, do not to forget to look at your patient. With today's technology, we can sometimes become lost in the monitors, the numbers, labs, vent settings, and those things are plentiful in the holistic picture, but looking at and observing your patient should not be lost in the shuffle, after all, that is whom we are taking care of.
Question and Answers
Are there ever instances where you would absolutely not use a neuromuscular blocking agent for intubation?
That is a great question. Well, I do not like to ever think of absolutes, but I will say that I certainly do my best to avoid them in patients who have a history of a difficult airway. For these patients, I might try to intubate with higher doses of hypnotic sedative agents like higher dose Propofol and avoid paralytic and take a look with a video scope or depending on the history, what do you mean to consider awake fiberoptic intubation, where I would use local anesthetics to numb up the airway and keep them breathing on their own. At the same time, I enter the trachea with a scope first. Another situation that I would avoid paralytics is in the case of an anterior mediastinal mass. Why? There's a potential for major airway and vascular or compression with these patients, and the paralytic dose could be fatal to them. Useful strategies to consider for these types of patients include awake fiberoptic intubation, maintenance of spontaneous ventilation as mentioned avoidance of muscle relaxants, intubation distal to the airway compression with the ENT team presence, positioning changes, immediate availability of rigid bronchoscopy, and you might even consider elective cardiopulmonary bypass in extreme cases. And then, of course, as mentioned in my talk, sometimes a patient is actively coding, and for those patients, they are usually already flaccid. There is no need for paralytics or drugs, period.
Are the muscle relaxants reversible and how often do you use these reverse agents?
I did not talk about the reversal muscle relaxants. It is a good question. The classic paralytics known as the nondepolarizers that I talked about are reversible. We use a drug called neostigmine, which is in the drug class known as cholinesterase inhibitors. These drugs indirectly increase the acetylcholine available to compete with whatever nondepolarizing agent we use for paralysis. If timed right, the reversal process optimizes within about seven to 12 minutes. Because of the side effects and neostigmine, one being bradycardia, we always couple it with a drug that counteracts this bradycardia, known as glycopyrrolate or ropinirole. There is some art form to all this, and I will not go into detail, but these drugs have to be adequately timed and given to optimize the patient's condition for extubation. We usually give this combination of reversal agents at the end of an operation where we are getting ready for the emergence of anesthesia. Another more recently introduced agent is Sugammadex, and this is a reversal more specific for rocuronium and vecuronium, both of which are nondepolarizers. Its mechanism of action works differently, but its popularity is definitely on the rise, and you do not have to couple it with another medication. As far as the reversible agents for Succinylcholine, I have been asked that question specifically, is there a reversal for Succinylcholine? All I can tell you is if the only thing that reverses it is time, and luckily, because it is a short duration of action, that time is not too long, about 8 to 10 minutes. This is a topic that we can discuss more with RTs and anesthesia collaborating specifically in critical care. I mean, there is still so much more information out there, and the gap between anesthesia and respiratory therapy in critical care is narrowing, and there must be more overlap of the knowledge base.
References
Arroyo-Novoa, C. M., Figueroa-Ramos, M. I., Balas, M., Rodríguez, P., & Puntillo, K. A. (2020). Opioid and Benzodiazepine Withdrawal Syndromes in Trauma ICU Patients: A Prospective Exploratory Study. Critical care explorations, 2(4), e0089. https://doi.org/10.1097/CCE.0000000000000089.
Conti, G., Mantz, J., Longrois, D., & Tonner, P. (2014). Sedation and weaning from mechanical ventilation: time for 'best practice' to catch up with new realities?. Multidisciplinary respiratory medicine, 9(1), 45. https://doi.org/10.1186/2049- 6958-9-45.
Kim, H. J., Kim, S. H., Min, N. H., & Park, W. K. (2016). Determination of the appropriate sizes of oropharyngeal airways in adults: correlation with external facial measurements: A randomised crossover study. European journal of anaesthesiology, 33(12), 936–942. https://doi.org/10.1097/EJA.0000000000000439.
Kuriyama, A., Jackson, J.L. & Kamei, J. Performance of the cuff leak test in adults in predicting post-extubation airway complications: a systematic review and meta- analysis.Crit Care 24, 640 (2020).
Lafferty, MD, K., Windle, PharmD, M., & Dillinger, R. (2019). What is the benefit of paralytic agents during tracheal intubation? Medscape. Published.
Morgan, G., Mikhail, M., & Murray, M. (2005). Clinical Anesthesiology, 4th Edition (4th ed.). McGraw-Hill Medical.
Nouruzi-Sedeh, P., Schumann, M., & Groeben, H. (2009). Laryngoscopy via Macintosh blade versus GlideScope: success rate and time for endotracheal intubation in untrained medical personnel. Anesthesiology, 110(1), 32–37. https://doi.org/10.1097/ALN.0b013e318190b6a7
Walls, R. M., Brown, C. A., 3rd, Bair, A. E., Pallin, D. J., & NEAR II Investigators (2011). Emergency airway management: a multi-center report of 8937 emergency department intubations. The Journal of emergency medicine, 41(4), 347–354. https://doi.org/10.1016/j.jemermed.2010.02.024
Citation
Vela, S. (2021). What’s anesthesia got to do with it? Continued.com - Respiratory Therapy, Article 94. Available at www.continued.com/respiratory-therapy
