Editor’s note: This text-based course is an edited transcript of the webinar Acute Respiratory Distress Syndrome (ARDS) and What You Should Know, presented by Tim Gilmore, PhD, RRT, RRT-ACCS, RRT-NPS, CPFT, AE-C.
It is recommended that you download the course handout to supplement this text format.
Learning Outcomes
After this course, participants will be able to:
- Define and describe acute respiratory distress syndrome in alignment with current Berlin criteria
- Discuss classifications of ARDS
- Explain evidence-based mechanical ventilation strategies as part of ARDS treatment.
Acute Respiratory Distress Syndrome
The goal is for you to walk away with practical knowledge you can apply immediately in your bedside practice. Ultimately, our goal is to enhance patient care. To start, let's take a brief look at the historical perspective. The term "acute respiratory distress syndrome" implies a sudden onset and a respiratory-specific condition, which is accurate. "Syndrome" usually refers to a recognizable complex of symptoms rather than a distinct disease. When we talk about a syndrome, we are referring to a set of physical findings that indicate a specific condition, though the direct cause may not be fully understood. We understand ARDS better now, and we know that certain factors can predispose an individual to develop it. However, there is still no definitive cause or predictable factor that will absolutely lead to ARDS. it is somewhat predictable but also unpredictable. We will delve deeper into this as we progress.
The condition of ARDS was first reported in 1967 in a publication in The Lancet by the renowned Dr. David Ashbaugh from Ohio State University. He identified a dozen patients in a case series who had a particular presentation or pathology with some common characteristics. The respiratory distress syndrome in 12 patients was manifested by acute onset of tachypnea, hypoxemia, and loss of compliance after a variety of stimuli; the syndrome did not respond to usual and ordinary methods of respiratory therapy. Positive end-expiratory pressure was most helpful in combating atelectasis and hypoxemia (Asbaugh et al., 1967).
What was most interesting about the early observations of this so-called syndrome was the presence of persistent refractory hypoxia, often without direct lung involvement. In other words, patients developed persistent and pervasive respiratory distress regardless of whether a direct lung insult was initially suspected. Almost all these patients exhibited some degree of pulmonary edema on chest X-rays despite not being in left ventricular failure. They showed pulmonary edema symptoms without obvious cardiovascular compromise. Most of the early cases involved younger, generally healthy servicemen during the Vietnam War in the late 1960s.
ARDS Defined
ARDS had numerous descriptions and borderline formal definitions from the 1970s through the '80s and even into the early '90s, but there was very little agreement among key groups. It was not until 1994 that ARDS was formally defined by the American-European Consensus Conference. This led to a panel of experts convened in 2011 by the European Respiratory Society and the Society of Critical Care Medicine. They developed what is now known as the Berlin Definition, which has become the gold standard for defining ARDS. This Berlin Definition is likely the most familiar to everyone today.
They met in Berlin, which is why it is called the Berlin Definition. Starting from the early years of defining ARDS in the 1990s to the early 2000s, the PF ratio (the partial pressure of arterial oxygen compared to the inspiratory fraction of oxygen) and various other clinical indicators of significant respiratory impairment have long been part of the criteria to officially diagnose ARDS. However, it was not until the European Society of Intensive Care Medicine, with endorsement from the American Thoracic Society and the Society of Critical Care Medicine, convened an international expert panel in Berlin that the ARDS definition was revised. This is how the Berlin Definition was created.
The Berlin Definition changed everything. In fact, it is one of the most cited articles in critical care medicine. I will mention early on that the term "acute lung injury" (ALI) has come and gone, although the concept remains relevant. Clinically, acute lung injury is still a viable concept. However, we will focus solely on ARDS during today's lecture. Now, let's examine the original Berlin Definition that made such a significant impact. Compared to the 1994 AECC definition, the Berlin Definition has better predictive validity for mortality. The emphasis is on early identification and preventing avoidable deaths.
The original Berlin Definition in Figure 1., included three mutually exclusive categories of ARDS based on the degree of hypoxemia, all determined by evaluating the PF ratio (the partial pressure of arterial oxygen compared to the inspiratory fraction of oxygen). These categories are:
- Mild ARDS: PF ratio greater than 200 but less than or equal to 300
- Moderate ARDS: PF ratio less than or equal to 200 but greater than or equal to 100
- Severe ARDS: PF ratio less than or equal to 100
However, diagnosing ARDS involves more than just assessing the PF ratio.
Berlin Definition of Acute Respiratory Distress Syndrome | |
Timing | Within 1 week of a known clinical insult or new or worsening respiratory symptoms |
Chest Imaging | Bilateral opacities - not fully explained by effusions, lobar/lung collapse, or nodules |
Origin of Edema | Respiratory failure is not fully explained by cardiac failure or fluid overload. Need objective assessment (e.g., echocardiography) to exclude hydrostatic edema if no risk factor present |
Oxygenation | Mild 200 mm Hg < Pa02/Fi02 ≤ 300 mm Hg with PEEP or CPAP ≥5 cm H20 Moderate 100 mm Hg < Pa02/Fi02 ≤ 200 mm Hg with PEEP ≥5 cm H20 Severe Pa02≥/Fi02 ≤ 100 mm Hg with PEEP ≥5 cm H20 |
| Abbreviations: CPAP, continuous positive airway pressure; Fi02, the fraction of inspired oxygen; Pa02, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure. Chest radiograph or computed tomography scan. If the altitude is higher than 1000m, the correction factor should be calculated as follows: [Pa02/Fi02X (barometric pressure/760)]. This may be delivered noninvasively in the mild acute respiratory distress syndrome group. JAMA, June 20, 2012—Vol 307, No. 23 |
Figure 1. Berlin Definition of ARDS.
As shown in Figure 1, timing is crucial. The term "acute" suggests that the onset occurs within a week or less. This rapid onset is significant. Additionally, a chest x-ray or CT scan is essential for diagnosis. These imaging techniques reveal opacities, also known as infiltrates, which must be present to confirm ARDS. Importantly, these thoracic infiltrates must originate from a non-cardiac source. Although heart failure can cause pulmonary edema, it does not classify as ARDS. The presence of true respiratory failure is assumed with ARDS. A key aspect of the classification process is the PF ratio, which categorizes the severity of the condition.
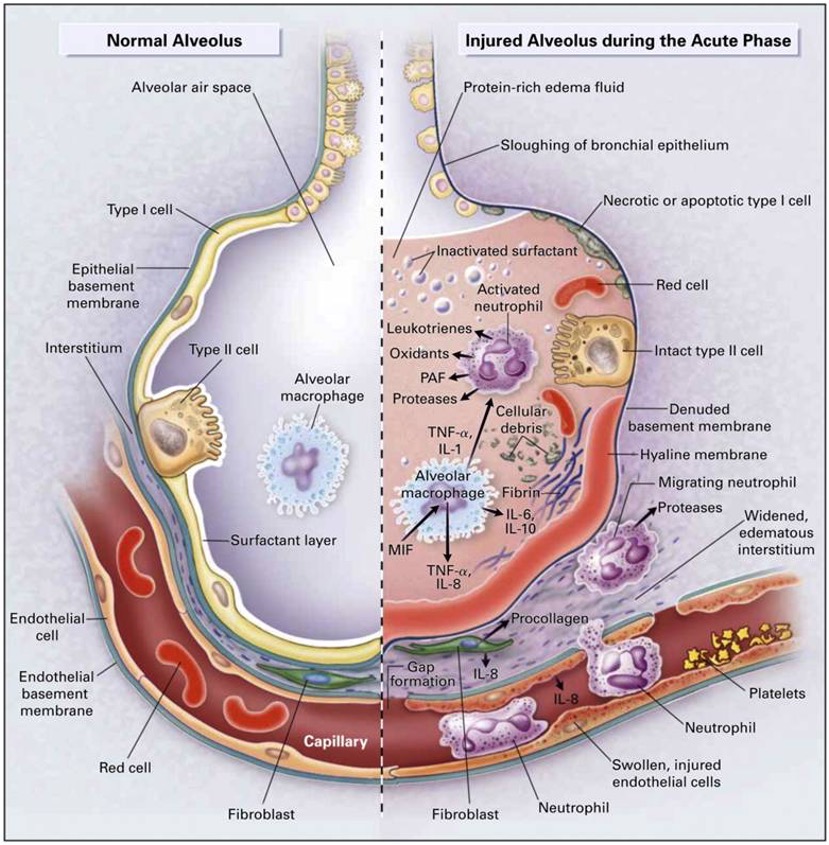
Figure 2. A diagram of the normal alveolus and injured alveolus during the acute ARDS phase (https://www.researchgate.net, CC BY-SA 4.0 via Wikimedia Commons).
Previously, the term "acute lung injury" (ALI) was used to describe a condition that could develop into ARDS. However, the ALI category is now obsolete. Today, we consider a patient to have ARDS if they meet specific clinical criteria. This is a clear, checkbox-based approach to diagnosis. ARDS, although somewhat unpredictable and sometimes discovered randomly, typically manifests after a significant infection or specific physical trauma. Importantly, this trauma does not necessarily need to directly involve the thorax. Figure 2, one of the more widely recognized images depicting ARDS, illustrates this well. On the far left of Figure 2, the key takeaway is that ARDS involves a severe inflammatory cascade. This cascade results in infiltrates and fluid accumulation in the lung acini, accompanied by a leaky pulmonary capillary bed. Platelets begin to aggregate, exacerbating the issues typically associated with an inflammatory response within the lung.
In Figure 2, you can see a comparison between normal alveoli on the left and injured alveoli on the right. The differences are striking. One critical aspect, which may not be clearly depicted in Figure 2, is the deactivation of surfactant. Normally, surfactant reduces surface tension in these tiny lung units. When it is washed out or deactivated, the lung becomes very stiff and non-compliant. Additionally, it is worth mentioning some of the common terminology associated with ARDS or ARDS-like conditions. These terms are often colloquial or clinical slang rather than standardized. However, our focus today is on the standard ARDS criteria.
ARDS stands for acute respiratory distress syndrome. As a side note, the term "baby lung" is often mentioned in the context of ARDS. "Baby lung" refers to the remaining healthy lung tissue that can still be ventilated when ARDS is present. Essentially, it is the functional lung tissue available for breathing. ARDS is considered a syndrome rather than a disease because there is no single known specific cause. it is not black and white. There are patterns and certain indications that a patient may be at risk for developing ARDS, but ultimately, it is a clinical syndrome diagnosed based on clinical features.
Here is a brief overview of those clinical features:
- Progressive dyspnea with increasing O2 requirements
- Alveolar infiltrates on chest x-ray & reduced SaO2 (SpO2) within 6-72h of inciting event
- Lab tests are nonspecific
Alveolar permeability presents a major challenge because it leads to intra-alveolar fluid accumulation, causing a ventilation-perfusion mismatch and right-to-left intrapulmonary shunting. This makes it extremely difficult to oxygenate these patients, who suffer from extremely low lung compliance. It is a vicious cycle: the stiffer the lung, the more creativity is required to ventilate. Otherwise, the cycle of alveolar infiltration continues.
In essence, ARDS can be summarized as sudden respiratory failure unrelated to heart failure or specific fluid overload. Patients present with pulmonary edema, but it is of non-cardiac origin. While there are other aspects to discuss, such as pulmonary capillary wedge pressure monitoring, today, we will focus on the essential items you need to know. Now, back to Figure 2. We know that ARDS pathophysiology is complex. Although it primarily affects the lungs, there is a significant risk of multi-organ involvement. In fact, much of the mortality associated with ARDS is due to multi-organ dysfunction syndrome (MODS). However, that is a topic for another day.
Before we move on, let's focus not only on the alveolar involvement depicted on the right side of Figure 2 but also on the intravascular and extravascular activities, also known as the interstitial processes. Notice the inflammatory mediators, such as interleukin, that are pervasive in both the alveoli and the capillaries. Due to increased capillary permeability, there is a massive fluid shift from inside the capillaries toward the interstitial space and into the gas exchange units. These patients are extremely difficult to oxygenate. Does this sound familiar to something We have encountered en masse over the last few years? The COVID-19 condition often presents similarly, making it challenging to differentiate between the pulmonary presentations of ARDS and COVID-19.
Epidemiology
When discussing any disease, disorder, or syndrome, it is helpful to quickly outline the epidemiology. Here is what we know. A well-done article by Hendrickson et al. (2021), published in the American Journal of Respiratory and Critical Care Medicine, reviewed 50 years of research and publications on ARDS. This makes it a fairly comprehensive source. What is mildly disturbing is the admission from the Canadian group of researchers, despite their bold statement that "No other intensive care syndrome has been as extensively studied as ARDS."
Many basic epidemiologic questions remain unsolved. In other words, the incidence, distribution, control methods, and overall contributing factors of this syndrome are not easily identified. We can define ARDS and occasionally recognize it, but piecing it all together remains challenging. The researchers cite a lack of standardization, and the exact onset of ARDS is not always easy to pinpoint. Even though our understanding of ARDS pathophysiology has certainly improved over the past five decades of published data, gaps remain. Several articles, not just this one, have referred to this extensive timeframe of available information.
Here is what we know: while pharmacologic interventions, such as corticosteroids and diuretics, have their place, the primary target of ARDS management should be the avoidance of ventilator-induced lung injury. We have a lot to consider, including noninvasive ventilation, high-flow nasal cannula, ECMO, and even extracorporeal CO2 removal, which we perform at my local facility. Before diving into these topics, it is important to quickly discuss the famous Lung Safe study by Bellani et al. (2016). Lung Safe stands for "Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure." This international, multi-center prospective cohort study included some well-known researchers and investigated patients undergoing invasive or non-invasive ventilation over four consecutive weeks nearly a decade ago.
The study included patients from 459 ICUs across 50 countries on five continents, resulting in a large sample size of just over 29,000 ICU admissions. Remarkably, over 10% of these patients met the criteria for ARDS. This highlights the prevalence of ARDS in ICU settings. If you work in an ICU, you will inevitably encounter ARDS. However, the study also revealed that we are not particularly good at recognizing or treating ARDS, which, unfortunately, remains associated with a high mortality rate. The landscape changed dramatically around 2020 with the onset of COVID-19. It is now clear that differentiating between ARDS and COVID-19 is challenging, as their pulmonary presentations, chest X-rays, lab results, and overall clinical manifestations are often indistinguishable.
Severe cases of COVID-19 are arguably more difficult to treat and require more creative approaches to both ventilator and overall clinical management. Nevertheless, ARDS criteria remain the same. The treatment of the respiratory side of things does not differ dramatically between COVID-19-induced ARDS and classic ARDS. There are over 60 known etiologies of ARDS. Hendrickson et al. (2019) listed some of the most common precursors to the development of this syndrome. If you work at a facility with high trauma cases or a large ICU patient census, you will encounter ARDS frequently. If you have worked in a hospital setting for any significant amount of time, you have likely seen most of these common precursors to ARDS: ARDS in various clinical settings.
- Sepsis
- Aspiration pneumonitis
- Infectious pneumonia (including mycobacterial, viral, fungal, parasitic)
- Severe trauma and/or multiple fractures
- Pulmonary contusion
- Burns and smoke inhalation
- Transfusion-related acute lung injury and massive transfusions
- HSCT
- Pancreatitis
- Inhalation injures other than smoke (e.g., near drowning, gases)
- Thoracic surgery (eg, post-cardiopulmonary bypass or other major surgery
- Drugs (chemotherapeutic agents, amiodarone, radiation)
Understanding these common precursors helps in recognizing and managing. If you work at a trauma center, you are likely to encounter ARDS more frequently than the average clinician. However, ARDS can develop in almost any patient, regardless of age. The official diagnosis is often unclear. There is a great variety of presentations, and no definitive diagnostic test exists to conclusively diagnose ARDS. While we can consider markers like C-reactive protein, no lab value consistently and sensitively alerts us to ARDS. The chest X-ray remains a key feature in the overall assessment of ARDS. At a minimum, a chest X-ray is required, though a CT scan would be ideal. Fluid specifically infiltrates the alveoli without any indication of cardiac failure or typical fluid overload. ARDS is a pulmonary condition, and although it is classified as a syndrome, it manifests as a pulmonary disease.
Stages of ARDS
- Early Exudative Stage (Early)
- Fibroproliferative Stage (Mid)
- Fibrotic Stage (Later)
As with any lung condition, ARDS typically follows an acute phase. While ARDS is characterized by rapid onset and unpredictability, it can be described in stages. These stages help in understanding the progression of the syndrome despite its acute nature. In the early stage, also known as DAD (diffuse alveolar damage), this occurs within the first 10 days of the process. This stage is not dissimilar to various other acute lung conditions. ARDS can also involve a prolonged recovery process. The histological hallmark of this early exudative stage is diffuse alveolar damage, characterized by edema and cellular infiltration, with cells migrating to the site of gas exchange. These cells gather in areas where they did not originate and should not reside.
The next stage, occurring beyond the first 10 days, is known as the fibroproliferative stage. In this stage, pulmonary edema resolves, but cellular infiltration and multiplication continue. Essentially, while the lung fluid decreases, cellular debris continues to accumulate. It is almost like leftover trash in the pulmonary system. The latter stage of ARDS is known as the fibrotic stage. Not all ARDS survivors necessarily progress to this stage, but many do. This stage is characterized by the deterioration of normal lung architecture and the presence of fibrosis or scarring. The extent of scarring varies, with some individuals experiencing minimal scarring while others suffer from severe scarring, requiring home oxygen for the rest of their lives, even after a short period of ARDS in the ICU. According to Dr. Yoshikawa, the subacute phase is a proliferative or organizing phase of ARDS In this phase, polyp-like spindle-shaped fibroblasts infiltrate the alveoli, further complicating the lung's recovery. Of course, anytime the distance between the free surface and the diffusible layer of the alveoli thickens, some degree of refractory hypoxemia is likely to develop. The red arrow in the image points to those spindle-shaped fibroblasts.
ARDS Mortality
To be clear, there is no magic potion or perfect combination of treatments for ARDS. Despite advances in technology and knowledge, the mortality rate remains extremely high. Maca et al. (2017) conducted a systematic review published in the Respiratory Care Journal, categorizing mortality rates by type, such as at 28 days, one month, 60 days, three months, and one year. From nearly 1,100 studies initially evaluated, about 177 papers were included.
Their conclusion was that reported mortality rates are highly variable among institutions, often biased by numerous factors. Without offering an exact number with confidence, we recognize that ARDS-related mortality rates remain high, typically ranging from 30% to 45%, depending on various factors. The highest mortality rate, unsurprisingly, occurs in the ICU. Patients who are critically ill enough to be admitted to the ICU are subject to the most intensive monitoring and diagnostic evaluation. An interesting study by Hendrickson et al. (2020) specifically identified adult patients hospitalized at a single tertiary care center over approximately one year. These patients had acute hypoxic respiratory failure, defined by a PF ratio of less than or equal to 300.
These patients met ARDS criteria while receiving invasive mechanical ventilation for at least 12 hours and ultimately died during hospitalization. The study focused on those who experienced mortality during their hospital stay. ARDS was verified by multiple physicians using the Berlin definition. Ketcham et al. (2020) studied under 400 patients and found that sepsis, pulmonary dysfunction, and neurologic dysfunction were the most commonly identified causes of death. Notably, 70% of those who died exhibited multi-organ failure at the time of death. This underscores the severe impact of ARDS.
Now, let's return to the broader medical community. Although there is rarely complete consensus worldwide, let's review the most updated Berlin criteria for ARDS. We will then quickly move into classification and conclude with some evidence-based ventilator strategies. The overarching summary is that ARDS has a rapid onset, typically less than a week. Chest X-rays will show bilateral infiltrates. Respiratory failure is present and is relatively unexplained by cardiac failure, indicating that the pulmonary edema is of non-cardiac origin. The PF ratio is decreased to 300 or below; normally, this number should be well above 400. In the oxygenation section, it is assumed that all patients are on a minimum PEEP of at least five centimeters of water pressure. This encapsulates ARDS in a nutshell. These criteria allow the ordering provider to code a patient with an ARDS diagnosis.
ARDS Redefined
Very recently, Villar et al. (2023) published a paper in Critical Care suggesting that we should further define or standardize the assessment of ARDS to include both pulmonary and extrapulmonary involvement. They argue that the PF ratio is insufficiently sensitive to guide treatment selection. I cannot disagree with that. They also include a compelling case study of a septic patient with a urinary tract infection. This patient was initially placed on a high-flow nasal cannula, then transitioned to invasive mechanical ventilation, but eventually improved and was discharged after a few days. The key question in their article was whether this patient truly had ARDS.
They contend that their patient initially met the ARDS criteria but quickly fell off after a short period of mechanical ventilation. According to the latest Berlin definition, which includes the PF ratio, their patient had ARDS. However, they question whether the patient truly had ARDS after showing slight improvement in mechanical ventilation. This points to the PF ratio not being the most sensitive indicator of true respiratory status.
Today’s topic is not meant to stir up controversy; we are simply examining the evidence. Villar et al. recommend using new data sets from 2010 and beyond, utilizing more current data. They also suggest that the PF ratio be calculated under more specific standard conditions, such as at predetermined FIO2 and PEEP levels. This approach aims to provide a more accurate assessment of respiratory status in ARDS patients. In other words, leveling the playing field with standardized conditions is not a bad idea. While theoretical concepts do not always easily translate into bedside practice, this one seems feasible. Let's dive into it.
We Are Sure It Is ARDS, Now What?
Let me reiterate that there is no magic potion or absolutely effective treatment for ARDS. We will focus on one of the most important aspects of therapeutic intervention for ARDS, which is mechanical ventilation. ARDS results in respiratory failure with complex oxygenation needs. Sometimes, a less-is-more approach may be acceptable in respiratory failure, but what about with ARDS? I hope to address this moving forward. Multi-organ failure is an unfortunate reality in the wake of ARDS. While not always, it occurs frequently. How do we avoid it? For starters, we seek to ventilate the lungs conservatively and protect the lung tissue as much as possible. A minimalist approach may be best.
Regardless of the journal, it is well established that ARDS and organ failure often go hand in hand, affecting more than just the lungs. Let's look at some strategies to manage this:
- Judicious Ventilation: Employing lung-protective ventilation strategies to minimize further lung injury.
- Minimalist Approach: Using the least amount of intervention necessary to support the patient.
- Organ Protection: Implementing measures to prevent or mitigate multi-organ failure.
By focusing on these aspects, we aim to improve outcomes for ARDS patients and reduce the incidence of multi-organ failure.
Mechanical Ventilation in ARDS
Many recommendations have been made over the years regarding the mechanical ventilation of ARDS patients. Let's focus on the evidence. A team from Vanderbilt (Fuller et al., 2015) published a review in Chest titled "Mechanical Ventilation in ARDS: A State-of-the-Art Review." This article, published over 15 years ago, remains one of the most cited articles of its time. They evaluated only high levels of evidence, specifically randomized control trials, including some of the most renowned trials of the day. In summary, a low tidal volume strategy with the maintenance of plateau pressure below 30 centimeters of water pressure, when possible, is the only method of positive pressure ventilation shown to date that improves survival.
They recommended several useful rescue therapies for severe hypoxemia, including high PEEP, often referred to as "Super PEEP" in some articles, alveolar recruitment maneuvers, and prone positioning. Those of you who regularly work in an ICU have likely seen these techniques integrated over the years for both ARDS and non-ARDS patients when managing severe hypoxemia refractory to usual care.
Do these recommendations, which are approaching 20 years old, still hold today? Let's take a closer look. Before we dive into more recommendations, it is important to note that this is a multi-center clinical trial (Fuller et al., 2015) conducted in collaboration among four academic medical centers, specifically their emergency departments.
Institution-specific influences and other potentially unaccounted-for confounding variables are always at play. Unfortunately, their conclusions may align with what we see at any given time in our emergency departments. Lung-protective ventilation is infrequently used in patients receiving mechanical ventilation in the ED, regardless of ARDS status. It is a concerning finding, especially since this study was conducted among four academic medical centers. We in academic medicine are supposed to be at the cutting edge and evidence-based, right? Yet, this is a reality many of us can relate to. The study also emphasized that the progression to ARDS is very common after admission to the emergency department. The title of the publication says it all.
Spontaneous Breathing
As I mentioned earlier, extensive research on ARDS spans over 50 years. To be clear, a publication by Yoshida et al. (2017) focused on whether ARDS patients, particularly those with severe cases, should be allowed to breathe spontaneously while on positive pressure ventilation. The group presents accumulating evidence that spontaneous breathing may cause or worsen acute lung injury, especially if the breathing is vigorous. We have all seen this type of patient.
In the end, the net impact of allowing ARDS patients to spontaneously breathe while on mechanical ventilation depends on the severity of the lung injury or the ARDS condition. The authors recommend ventilator strategies that either allow spontaneous breathing or use muscle paralysis during mechanical ventilation, depending on the stage and severity of ARDS.
A recent publication by Pelosi et al. (2021) in Critical Care outlines a personalized mechanical ventilation approach. This ongoing recommendation includes a low tidal volume strategy, with volumes as low as four milliliters per kilogram of predicted body weight being acceptable in some patients. There is also a focus on driving pressure, known as Delta P. Additionally, the use of an esophageal balloon to monitor pulmonary pressures, specifically pleural pressures, has well-established merit.
However, most experts argue that there are many barriers to acquiring accurate results and properly interpreting them. Even when the measurements are reliable, their fidelity can sometimes be questionable. While a personalized mechanical ventilation approach is theoretically ideal, implementing it in practice poses significant challenges. How do we truly attain this level of individualized care?
In 2021, a group of Italian physician researchers published what they called the "10 Golden Rules for Mechanically Ventilating ARDS Patients" (Battaglini, 2021). While not groundbreaking, it offers some valuable perspectives. First and foremost, an accurate diagnosis is essential before treating any patient. Classifying the severity of ARDS is also important. Key recommendations include maintaining conservative tidal volumes and minimal plateau pressures, as well as monitoring driving pressures (ΔP). PEEP is crucial, along with recruitment maneuvers and other strategies we have mentioned. Consider prone positioning for patients with refractory hypoxemia and extreme V/Q mismatching, as changing their position can allow gravity to help improve blood flow to the dependent regions of the lung.
- Maintaining conservative tidal volumes: Aim for low tidal volumes to prevent further lung injury.
- Minimal plateau pressures: Keep plateau pressures as low as possible.
- Monitoring driving pressures (Delta P): Focus on maintaining safe driving pressures.
- PEEP: Use appropriate positive end-expiratory pressure to keep the alveoli open.
- Recruitment maneuvers: Implement strategies to reopen collapsed alveoli.
Consider prone positioning for patients with refractory hypoxemia and extreme V/Q mismatching. Changing their position can allow gravity to aid in blood flow to dependent regions of the lung, which can be beneficial. These guidelines reinforce the importance of precise, patient-specific management strategies to optimize outcomes for ARDS patients.
- Classify severity
- Monitor and maintain Vt, Pplateau, ΔP
- Implement/exploit PEEP
- Utilize recruitment maneuvers
- In certain cases, implement paralyzation
- Decrease MV support when possible
- Use prone positioning
- Consider non-traditional rescue therapies as available (i.e., artificial lung, iNO)
- Consider ECMO
- Wean PPV when possible
ARDS-Related Complications
- Barotrauma
- Nosocomial infection (VAP/VAE)
- Delirium
- Thrombi
- GI bleeding
- Sleep disturbances
- Poor nutrition
- Multiorgan failure
If your institution has it, ECMO is a possibility, wean as soon as you can from mechanical ventilation. Almost every breath on positive pressure mechanical ventilation is antiphysiologic. As a side note, there is a relatively short yet well-known list of common mechanical ventilation-related complications encountered by ARDS patients. Many of these pathologies are also present outside the ARDS condition, but they are more commonly seen in ARDS patients, especially those experiencing mechanical ventilation and multi-organ failure. If you have worked with ARDS patients, you have likely seen these complications repeatedly. They almost develop into a pattern, with ARDS-related complications manifesting consistently.
Ventilate This!
Variables of PPV
- Flow
- Pressure
- Volume
- Time
This is a friendly reminder that we are dealing with extremely delicate lung tissue. When a surfactant is suddenly deactivated by the influx of fluid into the gas exchange spaces, you must be creative in avoiding underinflation and the risk of atelectrauma while also being cautious of hyperinflation and volume trauma. Studies suggest that both repeated cyclic opening and closing of lung units, as well as excessive driving pressure, contribute to the already significant pulmonary and systemic inflammation. Therefore, it is crucial to stabilize the lung unit and ventilate minimally.
When considering full inflation, bordering on hyperinflation, versus slight under-inflation, flirting with borderline atelectasis, the sweet spot lies somewhere in the middle. , how do you ventilate fluid-filled, stiff lungs? What is the takeaway? Given the multiplicity of modes, fine-tuned boutique settings, and proprietary options available to some but not to others, what can we do about mechanical ventilation? Clinicians vary in their openness to new methods. Some are evidence-based, while others rely on empirical experience. We have all encountered this variability. As a respiratory therapist, I understand the barriers, but there often comes a point when traditional options are exhausted, and oxygenation remains difficult. In those moments, even the most conservative caregivers become open to trying whatever may work.
I want to leave you with some evidence-based considerations. Ventilating many alveoli with various filling pressures and times, interconnected through the pores of Kohn and alveolar ducts, is a complex task. However, we can simplify it by focusing on the three main variables we can manipulate in real-time mechanical ventilation: flow, pressure, and volume. These variables are all plotted over time.
Flow and Volume
A ventilator is essentially a pressurized flow generator. Oversimplified, it is akin to pouring water into a bag; at its core, it is all fluid dynamics. The process of positive pressure ventilation today is almost identical to its original method, much like auscultation has not fundamentally changed over the years. Understanding these basics helps us navigate the complexities of mechanical ventilation more effectively.
Old Process, New Method
Thanks to technological advancements, the method has indeed evolved. For example, the original stethoscope involved simply placing your head on a patient to listen. The first formal stethoscope, invented in 1816 by French physician Rene Laennec, looks quite different from the modern stethoscope we use today. Similarly, with mechanical ventilation, we are still taking compressed gas and forcing flow under high velocity into a delicate system. While the basic process has not changed much over the decades, our methodologies certainly have.
Equation of Motion
- PVENT + PMUSC = (E x V) + (R x V)
- Pvent – pressure generated by ventilator > PEEP
- Pmusc – pressure generated by inspiratory muscles
- E – respiratory system elastance
- V – Δ lung volume above FRC
- R – respiratory system resistance
- V - flow
Consider Newton's equation of motion without getting too deep into the math. Think of acceleration as the derivative of velocity and velocity as the derivative of position. This understanding of dynamics helps us appreciate the intricate control we now have over mechanical ventilation settings.
Factors to “Overcome”
- Airways resistance (Raw)
- Lung Compliance (CL)
- Elasticity
- Thoracic Wall
In positive pressure ventilation, we must overcome the lung's elastic and resistive forces. The lung resists stretching, especially when the diaphragm is not contracting, and we are controlling each breath. Therefore, the ventilator and the respiratory musculature must work in harmony, particularly when a patient makes spontaneous efforts to encourage adequate ventilation and maintain synchrony.
As the term implies, these are challenges we must address while ventilating patients and each case is unique. According to Poiseuille's law, flow is indirectly proportional to the length of the tube and the viscosity of the gas. This means flow will decrease with a decrease in driving pressure or a decrease in tube radius. Understanding these principles helps us fine-tune our approach to mechanical ventilation for each patient.
Time Constant (T)
- 1 TC = CL x Raw
- How quickly pressure equilibrates between the circuit and alveoli
- 3-to-5-time constants required for complete inspiration/expiration
What is interesting is that even a small change in the cross-sectional area of an airway can dramatically alter the flow received into the lung due to its exponential relationship. Mathematically, if a patient's airway radius decreases by just 16%, the flow rate through that tube is cut in half. This sensitivity underscores the importance of maintaining airway patency.
Now, let’s talk about time constants. A time constant is the time required to fill a particular lung region to 63% of its potential capacity. Understanding this helps in managing ventilation, as different lung regions can have varying time constants, affecting how quickly they fill and empty. The goal is to target three to five-time constants for complete filling. In a normal lung, surface tension is not constant due to a mixture of phospholipids and proteins, known as surfactants, floating atop the alveolar epithelium. This surfactant helps gas flow from larger to smaller alveoli, producing homogeneity in alveolar volume and stabilizing the lung.
In ARDS, however, surfactant is deactivated by the mass infiltration of lung fluid, disrupting this balance. As a result, you can encounter very high airway resistance and, more critically, low compliance. When compliance decreases, as it does in ARDS, the time constant increases because it is a product of airway resistance and compliance. This is why an increase in inspiratory time is sometimes necessary to adequately recruit and oxygenate the lungs. Theoretically, if you allow enough time, even the most noncompliant lung can accept volume within reason. By extending the time, you enable a more equal distribution of gas, allowing the lung to accommodate the necessary volume despite its reduced compliance.
What About Ventilator Mode... Volume-Targeted or Pressure-Tageted?
This question is undoubtedly debated worldwide, let's examine some data. At face value, you could argue either side if you truly know how to manipulate the ventilator. However, many clinicians assume that pressure-targeted modes are more lung-protective, but let’s see what the evidence says. A systematic review and meta-analysis were conducted by Rittayam et al. (2015) to determine whether pressure-control continuous mandatory ventilation (PCCMV) or pressure-control inverse ratio ventilation (PCIRV) has demonstrated advantages over volume-controlled CMV. The Cochrane tool for risk of bias was used to assess methodological quality.
The researchers conducted their due diligence and introduced physiological criteria as quality indicators for selecting studies. They identified 34 studies that met the inclusion criteria and compared PCCMV and PCIRV to volume-control CMV. Their results indicated no significant differences in compliance or gas exchange, even with inverse ratio ventilation or when using pressure control with prolonged inspiratory time. They calculated the oxygen index and, after analyzing several patients, observed a poor effect of pressure-controlled inverse ratio ventilation on the oxygenation index in a large portion of patients. Additionally, there was no difference between the modes in terms of hemodynamics, work of breathing, or clinical outcomes. This suggests that the assumed superiority of pressure-targeted modes for lung protection may not hold true, as both modes showed comparable results across various parameters. Simply some things to think about.
Another systematic review by Chacko et al. (2015) evaluated acute lung injury in ARDS, analyzing over 60 years of data from studies conducted between 1950 and 2014. Their conclusions, which you can find in the article, indicate that currently available data from randomized controlled trials are insufficient to confirm or refute whether pressure-controlled or volume-controlled ventilation offers any advantage for patients with acute respiratory failure or ARDS. This suggests that while we may have some insights, the definitive answer remains elusive.
In 2000, the landmark ARDSNet study, a famous randomized controlled trial, demonstrated an approximate 9% absolute mortality reduction using a strategy of low tidal volume. This strategy has been well established since then, with a recommendation of 6 ml/kg of predicted body weight and a limitation of plateau pressure to less than 30 cm H2O as a starting point. This approach is considered ideal, though it represents just the minimum standard for managing ARDS patients.
However, current research suggests that driving pressure might be more crucial than simply focusing on tidal volume and plateau pressure. Driving pressure is the difference between peak inspiratory pressure and plateau pressure, or in pressure-control ventilation, the difference between inspiratory pressure and PEEP. This metric reflects the movement of lung tissue from rest to full inflation.
Gayat et al.'s (2018) article, related to the well-cited FROG study (French and European Outcome Registry study), evaluated 1,570 patients after discharge from the ICU. The term "ICU survivors" highlights that the ICU is indeed a dangerous environment, where patients are often more critically ill than average. The study underscores the importance of considering driving pressure as a significant factor in the management and outcomes of ICU patients, suggesting a need for a more nuanced approach to ventilation strategies beyond traditional metrics.
Ventilator Mismanagement Sign
ICU patients are already at higher risk for mortality upon admission. However, it has also been established that ICU survivors have decreased long-term survival compared to the general population, meaning they often die earlier, even after surviving an ICU stay. This highlights the need for careful management and consultation with pulmonary critical care specialists or respiratory therapists before placing a patient on a ventilator to avoid potential mismanagement.
This situation is sometimes referred to as the "ventilator mismanagement sign," which can occur right after intubation and the initiation of positive pressure ventilation. Always begin conservatively if possible. Where is the so-called sweet spot? The idea is to aim for a balance, finding a middle ground in ventilation strategies. This approach promotes optimal outcomes by avoiding the extremes of under- or over-ventilation and mitigating potential complications.
If you examine a microscopic view of alveoli under hyperinflation, you will see atelectasis and appreciate the dramatic differences between various levels of expansion. These alveoli are delicate, compressible, contractible, and recruitable lung units closely associated with adjacent capillaries that can also become recruited, dilated, constricted, collapsed, or compressed. Pulmonary blood flow is affected in real time as the alveoli expand and contract.
Finding the sweet spot of ideal inflation has been a long-standing concept. This so-called safe window of ventilation aims to avoid lung collapse at the end of expiration while preventing overfilling and overdistension during inspiration. The goal is to maintain this balance, ensuring optimal gas exchange without causing additional injury to the lung tissue.
Safe Window
To ensure this safe window, start with conservative tidal volumes. Typically, this means using 4 to 6 ml/kg of predicted body weight, occasionally up to 7 ml/kg, but preferably on the lower side to minimize lung injury. However, be mindful of hypercarbia, which can develop if you under-ventilate a patient. Permissive hypercapnia might be an option in some cases.
Monitor ventilating pressures carefully, whether you are using volume-targeting or pressure-targeting modes. Evaluate all the available scalars. Modern ventilators often provide a volume-pressure scalar, flow-volume loop, or other compliance curves, which can be evaluated quickly without adjusting settings or stimulating the patient. These tools help ensure you are within the safe window, balancing adequate ventilation and lung protection.
To evaluate a pressure-volume curve in its pure form, the patient should be in a flow-controlled mode with a very slow and prolonged inspiratory time. However, modern technology allows us to approximate these curves more easily. Evaluating lung mechanics in ARDS can be challenging, especially when determining the best PEEP. To generate an accurate pressure-volume curve, the patient must not make spontaneous efforts, and controlled flow delivery is typically achieved in a volume-targeting mode. However, the concept is more important than the specific method.
The goal is to identify two critical points:
- The pressure level at which the lung begins to rapidly fill with volume.
- The pressure level at which the lung begins to collapse on expiration.
By setting the PEEP just above the pressure level where the lung begins to collapse on expiration, you can prevent lung collapse, ensuring a more stable and effective ventilation strategy. To prevent ventilator-induced lung injury, we want to avoid the cyclic closing and reopening of alveoli with each breath. Since their lungs are already injured if they have ARDS, we should strive to minimize additional pulmonary inflammation whenever possible.
Here is a clinical tip: with the ordering provider's approval and supervision, you can occasionally step to the bedside and slightly increase the PEEP while observing the patient's chest rise. If you see an improvement in chest rise, an increase in oxygen saturation, and a sudden increase in expiratory volume (especially if you are in pressure control mode), you have likely recruited additional lung tissue.
There are other strategies to consider as well:
- Using recruitment maneuvers to open collapsed alveoli
- Adjusting inspiratory and expiratory times to optimize ventilation
- Monitoring for signs of overdistension to avoid further lung injury
By carefully adjusting these parameters, you can better manage ARDS patients and improve their respiratory outcomes. You can auscultate while slightly increasing the PEEP, allowing the patient to take a few breaths. If you notice an increase in aeration and air movement, it is likely that you have successfully recruited additional lung tissue. Sometimes, it requires a bit more creativity, such as adjusting inspiratory times and other settings, but this is a good starting point. In mechanical ventilation, we should be particularly concerned with the concepts of stress and strain. These terms, crucial in material science and engineering, are equally important in healthcare and medicine.
- Stress refers to the force applied to a material per unit area
- Strain is the deformation or change in the shape of the material resulting from the applied force
In the context of mechanical ventilation, stress corresponds to the pressure applied to the lung tissues, while strain represents the resulting changes in lung volume. Understanding and managing these concepts is essential to prevent ventilator-induced lung injury and optimize patient outcomes.
Generally Speaking…
- End-inspiratory plateau pressure and Vt/IBW ratio are inadequate surrogates to qualify and/or quantify end-inspiratory stress and strain Gattinoni et al., 2012)
- Large static strains are less harmful than large dynamic strains (Protti et al., 2013)
- An optimal “protective breath” describes one of minimal dynamic strain and energy/power load (Nieman et al., 2016)
- Lung protection > diaphragm protection (Goligher et al., 2020)
Within an alveolus, you can continue to increase pressure, applying stress without seeing any change in shape or recruitment. Only when you achieve a change in shape, through recruitment and increased volume in a particular lung unit, does the lung unit react to the applied force. Some of the most well-known names in clinical research support these concepts. Let's review the key points:
- Lower Tidal Volumes: Using lower tidal volumes is generally better for reducing lung injury.
- Lowest Possible Pressures: Maintaining the lowest pressures possible is crucial to minimize stress and strain on the lung tissues.
- Sustained Inflation: Sustained inflation (such as Super PEEP) is preferable to cyclic closing and reopening of alveoli. This approach reduces the risk of ventilator-induced lung injury.
This concept of sustained inflation aligns closely with the principles of high-frequency oscillatory ventilation (HFOV), which aims to maintain alveolar recruitment and minimize cyclic atelectasis and overdistension. Understanding and applying these principles can help optimize ventilation strategies and improve outcomes for patients with ARDS and other forms of acute lung injury.
The most gentle delivery possible, as tolerated by the patient, is ideal. However, this is often easier said than done. Recently, there has been much debate about whether to allow spontaneous breathing during the more severe stages of ARDS. The consensus seems to be shifting towards prioritizing lung protection over maintaining diaphragm activity, at least in the short term during the most severe phase of ARDS.
If a patient is asynchronous in deteriorating and unable to oxygenate due to issues like clamping down, then removing spontaneous efforts from the equation may be necessary. This approach can help ensure more consistent and controlled ventilation, potentially improving oxygenation and reducing the risk of further lung injury. Ultimately, the goal is to balance lung protection with overall patient stability, adapting strategies as the patient's condition evolves.
Summary
- Lung protective ventilation is a must
- Lower tidal volumes
- Application of optimal (and adequate) PEEP may require higher than traditional settings
- Target low plateau pressure (and low driving pressure)
- Use recruitment maneuvers cautiously
- Consider prone positioning
- Seek to maintain ventilator synchrony – may require neuromuscular blockade
- ***Don’t disregard potential non-traditional intervention as an effective option: NIV, HFOV, APRV, ECMO (Liaqat et al., 2022)
Here are some key points to take away:
- Lung Protective Ventilation: This is essential and begins with using conservative tidal volumes. Even in pressure-control or pressure-targeted modes of ventilation, it is crucial to monitor tidal volumes alongside plateau pressure.
- Adequate PEEP: Finding the optimal PEEP is vital. This "sweet spot" varies for each patient and is dynamic, meaning the PEEP setting at the start of a shift may not be appropriate later on. Regularly reassess and adjust PEEP as needed throughout the day.
- Monitoring and Adaptation: Continuous monitoring and adaptability are key. Pay attention to how the patient's condition evolves and be ready to adjust ventilation settings accordingly to maintain lung protection and optimize oxygenation.
- Balancing Lung Protection and Diaphragm Function: While lung protection is a priority, especially in the severe stages of ARDS, sometimes it may be necessary to eliminate spontaneous breathing efforts if they cause significant asynchrony and impede oxygenation.
By adhering to these principles, you can help minimize ventilator-induced lung injury and improve patient outcomes.
- Recruitment Maneuvers:
- Use Cautiously: Recruitment maneuvers can be beneficial but should be implemented cautiously.
- Open Lung Technique: This technique, which involves applying extremely high pressure for a short period to recruit alveoli, has been around for several years. However, it is less promoted in recent literature, with a preference for simpler recruitment strategies based on scalars and clinical monitoring.
- Prone Positioning:
- Team Effort: Prone positioning requires coordination and buy-in from the ordering provider.
- Quick Transition: When done correctly, it only takes a few moments to move a patient from supine (facing up) to prone (facing down). This position can significantly improve oxygenation and lung mechanics.
By integrating these strategies, you can enhance ventilation management, reduce lung injury, and improve patient outcomes.:
- Driving Pressure: Focus on maintaining a safe driving pressure to minimize lung injury.
- Recruitment Maneuvers: Implement these with caution and base them on clinical monitoring.
- Prone Positioning: Consider this effective strategy, especially for patients with severe ARDS, as it can significantly improve outcomes.
Monitoring for skin breakdown, lines, and other potential issues is crucial when implementing prone positioning, but the benefits can be significant. I recently saw a patient with ARDS who had reached the end of conventional ventilator options, and their oxygenation status improved dramatically upon being placed in the prone position.
- Key Points to Consider:
- Prone Positioning:
- Monitor for Skin Breakdown: Regularly check for skin breakdown, dislodged lines, and other complications.
- Significant Impact: Prone positioning can rapidly improve oxygenation status in patients with severe ARDS.
- Maintaining Synchrony:
- Neuromuscular Blockade is sometimes necessary to eliminate spontaneous breathing efforts and ensure ventilator synchrony. There is supporting literature for this approach.
- Alternative Ventilation Strategies:
- Non-Invasive Ventilation (NIV): Useful in certain scenarios, particularly if the primary issue is oxygenation rather than ventilation.
- High-Frequency Airway Pressure Release Ventilation (HFAPRV): Another option for specific cases.
- ECMO: Considered for severe cases where other interventions fail.
- High-Flow Nasal Cannula (HFNC): Can be effective for oxygenation problems without significant ventilation issues.
- Prone Positioning:
This is indeed a broad but vital topic in respiratory care and clinical medicine. By employing these strategies thoughtfully, we can improve patient outcomes and manage complex cases of ARDS and other respiratory conditions more effectively.
Questions and Answers
Do you find that a high-flow nasal cannula is helpful early on in the weaning process from the ventilator?
A high-flow nasal cannula (HFNC) can be very helpful, and I can tell you why. There are two trains of thought here. With HFNC, you can deliver a massive amount of flow with high FIO2s. This can be particularly beneficial in cases of refractory hypoxemia where lung recruitment is not an immediate priority, and the patient is simply in a distressed state. HFNC allows the patient to calm down and may decrease their oxygen consumption, giving you time to perform a better assessment. Not every patient will respond to HFNC, and once they deteriorate and become truly refractory, they will need positive pressure ventilation. However, if you have the option at your institution, trying HFNC early is worthwhile unless you know the patient is in a more severe ARDS condition. With limited information, it is best to try oxygen first. I always recommend trying high levels of oxygen flow before resorting to mechanical ventilation unless you have a chest X-ray and a good clinical history indicating otherwise.
What additional metrics would you find valuable at the bedside to quantify inspiratory stress and strain? For example, if you're closely monitoring driving pressure or dynamic strain based on compliance, what parameters seem easiest to capture and interpret at the bedside?
I wish I had a definitive list, too; it would probably have me giving talks around the world. However, over the years, I have noticed a few key insights beyond what We have discussed today. When you look at the compliance curve or the volume-pressure scalar, you can identify what used to be called the "bird's beak" or the point of overdistention. This is where you see a flattening of the curve after a progressive, almost linear increase in pressure and volume. Suddenly, with each unit increase in pressure, there is very little to no change in volume. This indicates you're overstretching the lung tissue, and it is crucial to avoid this. Not all ventilators provide scalar data with enough detail for precise assessment, but you can still make informed decisions. As you increase the PEEP or other pressure settings, watch for a point where the return volume increases and then suddenly stops. Continuing to increase pressure beyond this point is likely not beneficial. Sometimes, after recruiting lung tissue, you can slightly reduce the pressure while maintaining lung recruitment. Other times, it might not be as straightforward. This is where your assessment skills are essential. Monitor chest rise, the patient’s degree of comfort, the absence of grimacing (if they looked distressed before), oxygen saturation, blood pressure, and heart rate. Simple indicators like these can help you determine if the patient is responding positively. When making changes, observe if they are improving or heading in the right direction. The patient’s response can often guide you better than any scalar or curve alone.
When you're looking at sustained plateau pressures over 30 despite the usual interventions, what other considerations come into play?
One of the first things that come to mind for refractory hypoxemia is permissive hypercapnia. If we are really concerned about their oxygenation level, reducing O2 consumption is key. For instance, using a cooling blanket or even packing the patient in ice in extreme scenarios can help lower their metabolic rate. Administering a paralytic can also reduce O2 consumption in hypermetabolic patients, decreasing the lung's demand for oxygen. Additionally, proning the patient or maintaining sustained pressure for longer periods can help. This technique essentially splints the lung tissue open, promoting more equal distribution of gases and allowing oxygen molecules to diffuse more efficiently across the alveolar membrane. Monitoring fluid status is another critical aspect. Many patients are underhydrated, and ensuring they are adequately hydrated can improve their oxygenation status. In some cases, making the patient hyperdynamic can be beneficial, provided their cardiovascular system is not compromised. Potential pulmonary edema can be managed with diuretics later if necessary. Anti-inflammatories, particularly corticosteroids, are also frequently discussed in the literature. While they are not a cure-all, they can help manage inflammation, although they may not have an immediate impact on oxygenation status. By integrating these strategies—reducing oxygen consumption, optimizing gas distribution, ensuring proper hydration, and managing inflammation—clinicians can better address refractory hypoxemia and improve patient outcomes in severe respiratory distress.
References
ARDS Definition Task Force; Ranieri, V. M., Rubenfeld, G. D., Thompson, B. T., Ferguson, N. D., Caldwell, E., Fan, E., Camporota, L., & Slutsky, A. S. (2012). Acute respiratory distress syndrome: The Berlin Definition. JAMA, 307(23), 2526-2533.
Ashbaugh, D., Boyd Bigelow, D., Petty, T., & Levine, B. (1967). Acute respiratory distress in adults. The Lancet, 290(7511), 319-323.
Battaglini, D., Sottano, M., Ball, L., Robba, C., Rocco, P. R. M., & Pelosi, P. (2021). Ten golden rules for individualized mechanical ventilation in acute respiratory distress syndrome. Journal of Intensive Medicine, 1(1), 42-51.
Bellani, G., Laffey, J. G., Pham, T., Fan, E., Brochard, L., Esteban, A., Gattinoni, L., van Haren, F., Larsson, A., McAuley, D. F., Ranieri, M., Rubenfeld, G., Thompson, B. T., Wrigge, H., Slutsky, A. S., & Pesenti, A.; LUNG SAFE Investigators; ESICM Trials Group. (2016). Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA, 315(8), 788-800. doi: 10.1001/jama.2016.0291. Erratum in: JAMA, 316(3), 350.
Fuller, B. M., Mohr, N. M., Miller, C. N., Deitchman, A. R., Levine, B. J., Castagno, N., Hassebroek, E. C., Dhedhi, A., Scott-Wittenborn, N., Grace, E., Lehew, C., & Kollef, M. H. (2015). Mechanical ventilation and ARDS in the ED: A multicenter, observational, prospective, cross-sectional study. Chest, 148(2), 365-374.
Gattinoni, L., Carlesso, E., & Caironi, P. (2012). Stress and strain within the lung. Current Opinion in Critical Care, 18(1), 42-47.
Goligher, E. C., Dres, M., Patel, B. K., Sahetya, S. K., Beitler, J. R., Telias, I., Yoshida, T., Vaporidi, K., Grieco, D. L., Schepens, T., Grasselli, G., Spadaro, S., Dianti, J., Amato, M., Bellani, G., Demoule, A., Fan, E., Ferguson, N. D., Georgopoulos, D., Guérin, C., Khemani, R. G., Laghi, F., Mercat, A., Mojoli, F., Ottenheijm, C. A. C., Jaber, S., Heunks, L., Mancebo, J., Mauri, T., Pesenti, A., & Brochard, L. (2020). Lung- and diaphragm-protective ventilation. American Journal of Respiratory and Critical Care Medicine, 202(7), 950-961.
Hendrickson, K. W., Peltan, I. D., & Brown, S. M. (2021). The epidemiology of acute respiratory distress syndrome before and after Coronavirus Disease 2019. Critical Care Clinics, 37(4), 703-716.
Ketcham, S. W., Sedhai, Y. R., Miller, H. C., Bolig, T. C., Ludwig, A., Co, I., Claar, D., McSparron, J. I., Prescott, H. C., & Sjoding, M. W. (2020). Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: A retrospective cohort study. Critical Care, 24(1), 391.
Liaqat, A., Mason, M., Foster, B. J., Kulkarni, S., Barlas, A., Farooq, A. M., Patak, P., Liaqat, H., Basso, R. G., & Zaman, M. S. (2022). Evidence-based mechanical ventilatory strategies in ARDS. Journal of Clinical Medicine, 11(2), 319.
Máca, J., Jor, O., Holub, M., Sklienka, P., Burša, F., Burda, M., Janout, V., & Ševčík, P. (2017). Past and present ARDS mortality rates: A systematic review. Respiratory Care, 62(1), 113-122.
Matthay, M. A., & Zimmerman, G. A. (2005). American Journal of Respiratory Cell and Molecular Biology, 33, 319-327.
McGurk, K., Riveros, T., Johnson, N., & Dyer, S. (2020). A primer on proning in the emergency department. Journal of the American College of Emergency Physicians Open, 1(6), 1703-1708. doi: 10.1002/emp2.12175.
Nieman, G. F., Satalin, J., Andrews, P., Habashi, N. M., & Gatto, L. A. (2016). Lung stress, strain, and energy load: Engineering concepts to understand the mechanism of ventilator-induced lung injury (VILI). Intensive Care Medicine Experimental, 4(1), 16.
Pelosi, P., Ball, L., Barbas, C. S. V., Bellomo, R., Burns, K. E. A., Einav, S., Gattinoni, L., Laffey, J. G., Marini, J. J., Myatra, S. N., Schultz, M. J., Teboul, J. L., & Rocco, P. R. M. (2021). Personalized mechanical ventilation in acute respiratory distress syndrome. Critical Care, 25(1), 250.
Pham, T., & Rubenfeld, G. D. (2017). Fifty years of research in ARDS. The epidemiology of acute respiratory distress syndrome: A 50th birthday review. American Journal of Respiratory and Critical Care Medicine, 195(7), 860-870.
Protti, A., Andreis, D. T., Monti, M., Santini, A., Sparacino, C. C., Langer, T., Votta, E., Gatti, S., Lombardi, L., Leopardi, O., Masson, S., Cressoni, M., & Gattinoni, L. (2013). Lung stress and strain during mechanical ventilation: Any difference between statics and dynamics? Critical Care Medicine, 41(4), 1046-1055.
Villar, J., Szakmany, T., Grasselli, G., & Camporota, L. (2023). Redefining ARDS: A paradigm shift. Critical Care, 27(1), 416.
Citation
Gilmore, Tim (2023). Acute respiratory distress syndrome (ARDS) and what you should know. Continued.com - Respiratory Therapy, Article 228. Available at www.continued.com/respiratory-therapy